Titanium cobalt catalyst for photothermal chemical cycle decomposition of CO2 and preparation method thereof
A cobalt catalyst and photothermochemical technology, applied in the field of catalysis, can solve problems that need to be improved, and achieve the effects of excellent photoresponse, high activity, and good photoresponse ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] According to the general formula Co of titanium cobalt catalyst x Ti 1-x o 2-x , set x=0.15, the simplified chemical formula is Co 0.15 Ti 0.85 o 1.85 .
[0032] According to the general formula Co x Ti 1-x o 2-x , where x=0.15, weigh CoSO according to the molar ratio 4 ·7H 2 O and TiOSO 4 , and then according to the molar ratio TiOSO 4 :C 6 h 8 o 7 ·H 2 O is 1:3 and weighs citric acid C 6 h 8 o 7 ·H 2 O, add deionized water to 80 ℃ water bath and heat until completely dissolved, TiOSO in the solution 4 The molar concentration is 0.5mol / L.
[0033] Add 5 mol / L NaOH solution dropwise to adjust the pH to 9, and continue to stir for 30 minutes to obtain a gel-like mixture; transfer it to a stainless steel hydrothermal kettle lined with polytetrafluoroethylene, and heat it at a heating rate of 2°C / min to 160°C and keep warm for 24h; centrifuge the resulting mixture at 5000r / min for 10min, remove the insoluble matter in the lower layer, and wash with abs...
example 2
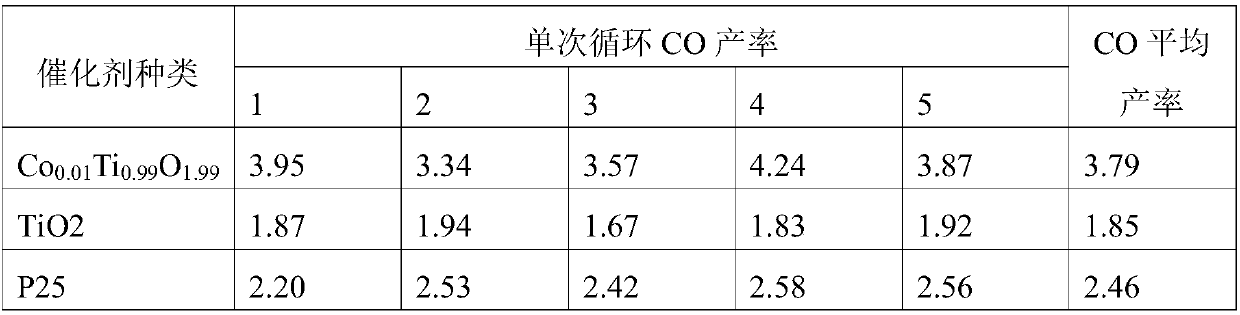
[0035] According to the general formula Co of titanium cobalt catalyst x Ti 1-x o 2-x , set x=0.01, the simplified chemical formula is Co 0.01 Ti 0.99 o 1.99 .
[0036] According to the general formula Co x Ti 1-x o 2-x , where x=0.01, weigh CoSO according to the molar ratio 4 ·7H 2 O and TiOSO 4 , and then according to the molar ratio TiOSO 4 :C 6 h 8 o 7 ·H 2 O is 1:3 and weighs citric acid C 6 h8 o 7 ·H 2 O. Add the three substances into deionized water, heat in a water bath at 80°C until completely dissolved, and TiOSO in the solution 4 The molar concentration is 0.3mol / L.
[0037] Add 7mol / L NaOH solution dropwise to adjust the pH to 9.5, and continue to stir for 30 minutes to obtain a gel-like mixture; transfer it to a polytetrafluoroethylene-lined stainless steel hydrothermal kettle, and heat it at a heating rate of 2.5°C / min to 160°C and keep warm for 24h; centrifuge the obtained mixture at a speed of 5000r / min for 10min, remove the insoluble matte...
example 3
[0039] According to the general formula Co of titanium cobalt catalyst x Ti 1-x o 2-x , set x=0.3, the simplified chemical formula is Co 0.3 Ti 0.7 o 1.7 .
[0040] According to the general formula Co x Ti 1-x o 2-x , where x=0.3, weigh CoSO according to the molar ratio 4 ·7H 2 O and TiOSO 4 , and then according to the molar ratio TiOSO 4 :C 6 h 8 o 7 ·H 2 O is 1:3 and weighs citric acid C 6 h 8 o 7 ·H 2 O, add deionized water to 80 ℃ water bath and heat until completely dissolved, TiOSO in the solution 4 The molar concentration is 0.2mol / L.
[0041] Add 10 mol / L NaOH solution dropwise to adjust the pH to 10, and continue to stir for 30 minutes to obtain a gel-like mixture; transfer it to a stainless steel hydrothermal kettle lined with polytetrafluoroethylene, and heat it at a heating rate of 3°C / min to 160°C and keep warm for 24h; centrifuge the obtained mixture at a speed of 5000r / min for 10min, remove the insoluble matter in the lower layer, and wash w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com