NIR-II conjugated nano particles, and preparation method and application thereof
A nanoparticle and near-infrared technology, applied in wave energy or particle radiation treatment materials, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve problems such as limiting large-scale preparation and clinical transformation, and achieve good clinical transformation possibilities. The effect of stability, mild reaction system and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Conjugated Small Molecules
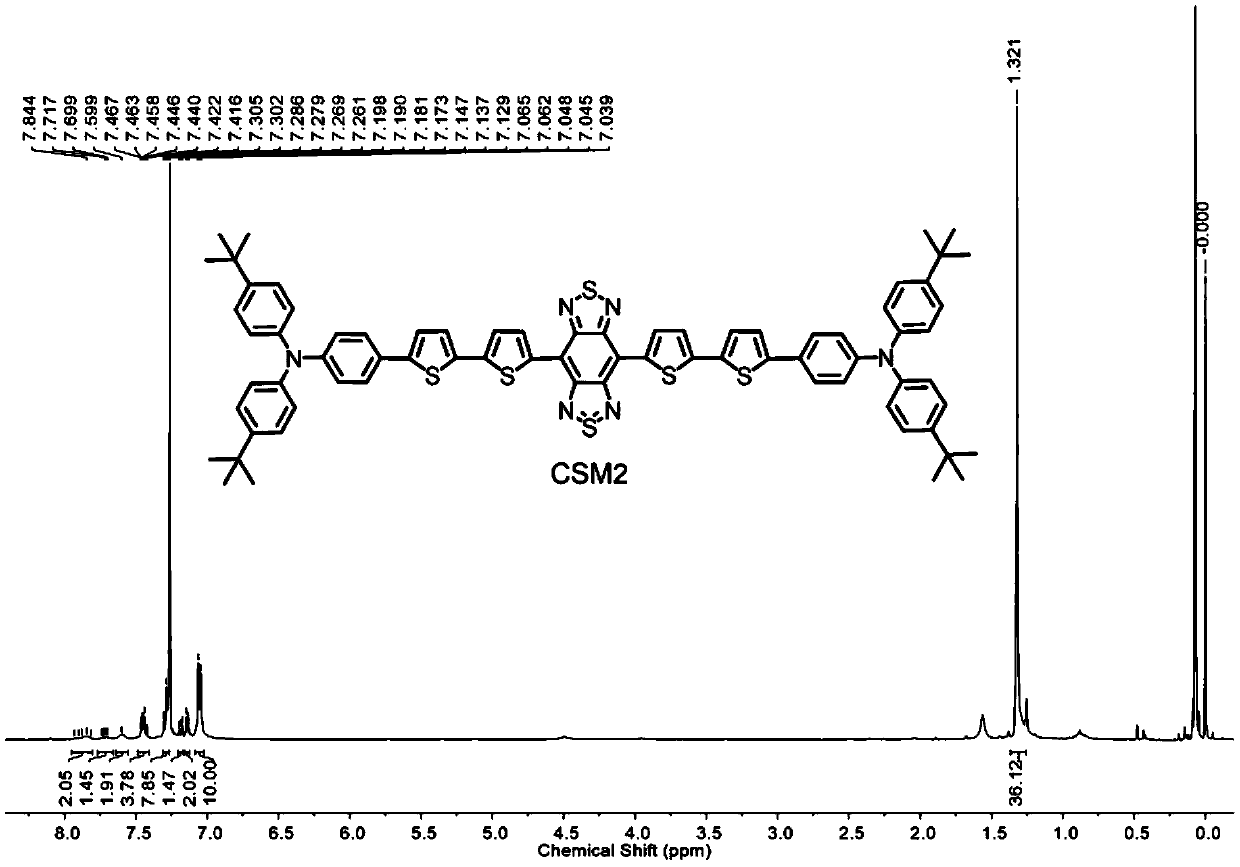
[0046] Dibromobenzobisthiadiazole (88.0mg, 0.25mmol), 4-tert-butyl-N-(4-tert-butylphenyl)-N-(4-(5'-trimethyltinyl) -(2,2'-Bithienyl)-5-phenyl)aniline (342.3 mg, 0.50 mmol) and bis(triphenylphosphine)palladium dichloride (8.77 mg, 0.0125 mmol) were dissolved in 30 mL of dry toluene , refluxed for 24 hours under an inert atmosphere, and finally silica gel column chromatography (dichloromethane as the eluent) obtained a conjugated small molecule in the second near-infrared region with a yield of 60%.
[0047] The obtained conjugated small molecules in the second near-infrared region were characterized by proton nuclear magnetic resonance spectroscopy and MALDI-TOF mass spectrometry. The results are shown in the attached figure 1 And attached figure 2 shown.
Embodiment 2
[0048] Example 2: Conjugated Small Molecules
[0049] Dibromobenzobisthiadiazole (88.0mg, 0.25mmol), 4-tert-butyl-N-(4-tert-butylphenyl)-N-(4-(5'-trimethyltinyl) -(2,2'-Bithienyl)-5-phenyl)aniline (342.3 mg, 0.50 mmol) and tetrakis(triphenylphosphine)palladium (14.4 mg, 0.0125 mmol) were dissolved in 30 mL of dry toluene, inert Reflux under the atmosphere for 24 hours, and finally silica gel column chromatography (eluent is dichloromethane) to obtain the conjugated small molecule in the second near-infrared region with a yield of 65%.
Embodiment 3
[0050] Example 3: Conjugated Small Molecules
[0051] Dibromobenzobisthiadiazole (88.0mg, 0.25mmol), 4-tert-butyl-N-(4-tert-butylphenyl)-N-(4-(5'-trimethyltinyl) -(2,2'-Bithienyl)-5-phenyl)aniline (342.3 mg, 0.50 mmol) and bis(dibenzylideneacetone)palladium (7.19 mg, 0.0125 mmol) were dissolved in 30 mL of dry toluene, Reflux for 24 hours under an inert atmosphere, and finally silica gel column chromatography (dichloromethane as the eluent) to obtain a conjugated small molecule in the second near-infrared region with a yield of 61%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com