A method for detecting related substances in chloral hydrate
A technology for chloral hydrate and related substances, which is applied to measurement devices, instruments, scientific instruments, etc., can solve the problems of undiscovered chloral hydrate raw materials and related preparations, and achieves the effect of ensuring clinical efficacy, high sensitivity, and high analysis efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
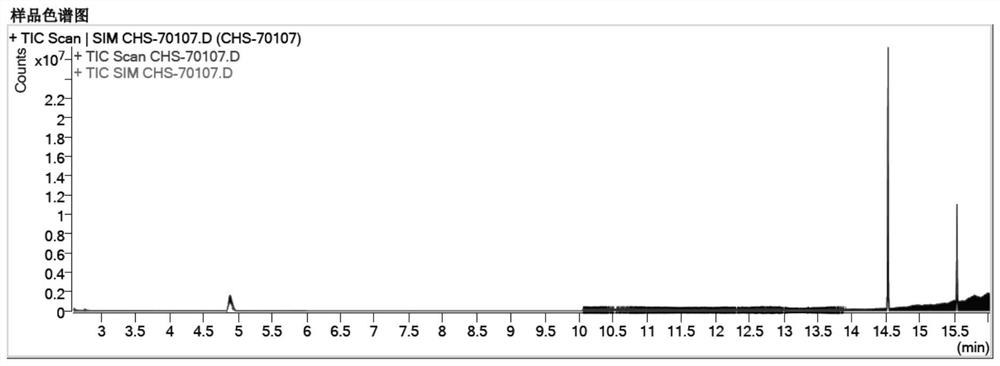
[0068] Embodiment 1, determination of related substances of chloral hydrate suppository
[0069] The instrument uses Agilent 7890B gas chromatograph + Agilent 5977B mass spectrometer detector; METTLER XP205 electronic balance.
[0070] The material is the chloral hydrate suppository prepared by the technology center of our company and the chloral hydrate suppository of Japan Hisamitsu Pharmaceutical;
[0071] The formic acid reference substance was purchased from Anaqua Chemicals Supply, the batch number was 73C1512FE; the chloroform reference substance was purchased from Anaqua Chemicals Supply, the batch number was 38Y1504MA; the trichloroethanol reference substance was purchased from Aladdin, the batch number was C1512073; the dichloroacetic acid reference substance was purchased from Aladdin, The batch number is K1715066; the trichloroacetic acid reference substance was purchased from Aladdin, the batch number is K1713111;
Embodiment 2
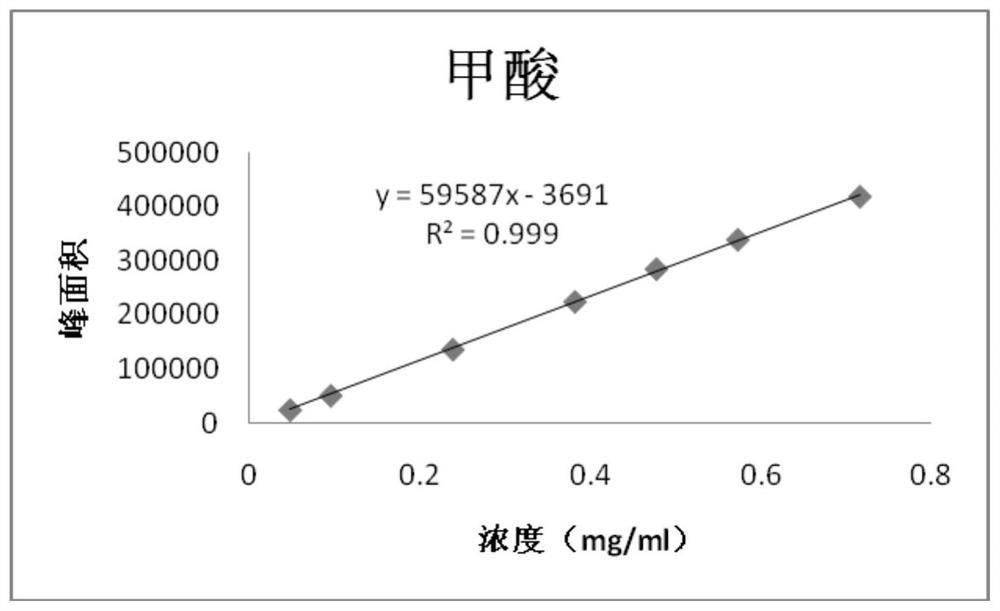
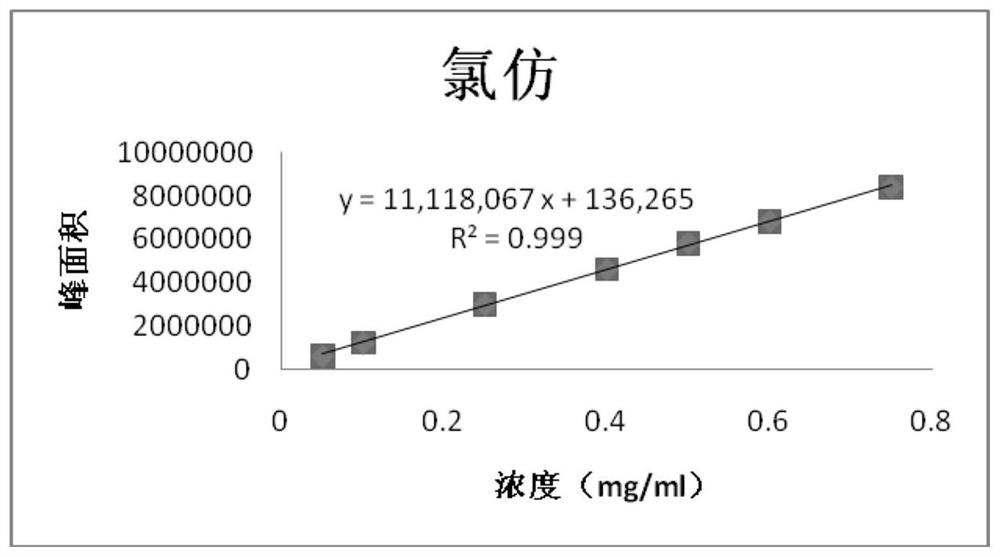
[0122] Embodiment 2, linearity test
[0123] 1. Prepare a series of impurity reference substance solutions with different concentrations
[0124] Precisely measure 1, 2, 2, 4, 2, 6, 3ml of impurity reference substance stock solution (2.5mg / ml), respectively put them in 50, 50, 20, 25, 10, 25, 10ml measuring bottles, add ethanol Dilute to the mark and shake well to obtain the linear test solution for impurities of each concentration.
[0125] 2. Carry out GC-MS detection of each impurity reference substance solution, and the detection conditions are as follows:
[0126] Detection condition is all the same as embodiment 1
[0127] After the GC-MS detection is completed, record the peak area of each impurity and each concentration of the reference substance, with the concentration as the independent variable and the corresponding peak area as the dependent variable, to obtain a linear regression equation:
[0128] The obtained results are shown in Tables 6-10;
[0129] Tabl...
Embodiment 3
[0144] Embodiment 3, specificity test
[0145] 1. Preparation of the test solution
[0146] With embodiment 1;
[0147] 2. Preparation of each impurity reference substance solution
[0148] With embodiment 1;
[0149] 3. Preparation of negative control solution
[0150] Accurately weigh about 400 mg of the blank of the content of the chloral hydrate suppository, and put it in a 25 ml stoppered colorimetric tube, accurately add 2 ml of ethanol, 2 ml of 20% sulfuric acid ethanol solution (V / V), 0.1 g of anhydrous magnesium sulfate, and close the plug. Vortex to dissolve, heat in a 50°C water-bath shaker for 40 minutes, take it out and quickly shower, add 4ml of n-heptane precisely, vortex and mix, add 20ml of water, shake, stand for stratification, and take the supernatant as Negative control solution.
[0151] 4. Determination of impurity content in the test solution and negative control solution
[0152] Carry out GC-MS analysis to above-mentioned impurity reference subs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| coating thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com