Preparation method for Itraconazole composite particles
A technology of itraconazole and composite particles, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, can solve problems such as unfavorable production scale-up, and achieves favorable dispersion and safe formulation. Stable and effective, easy to produce and scale up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
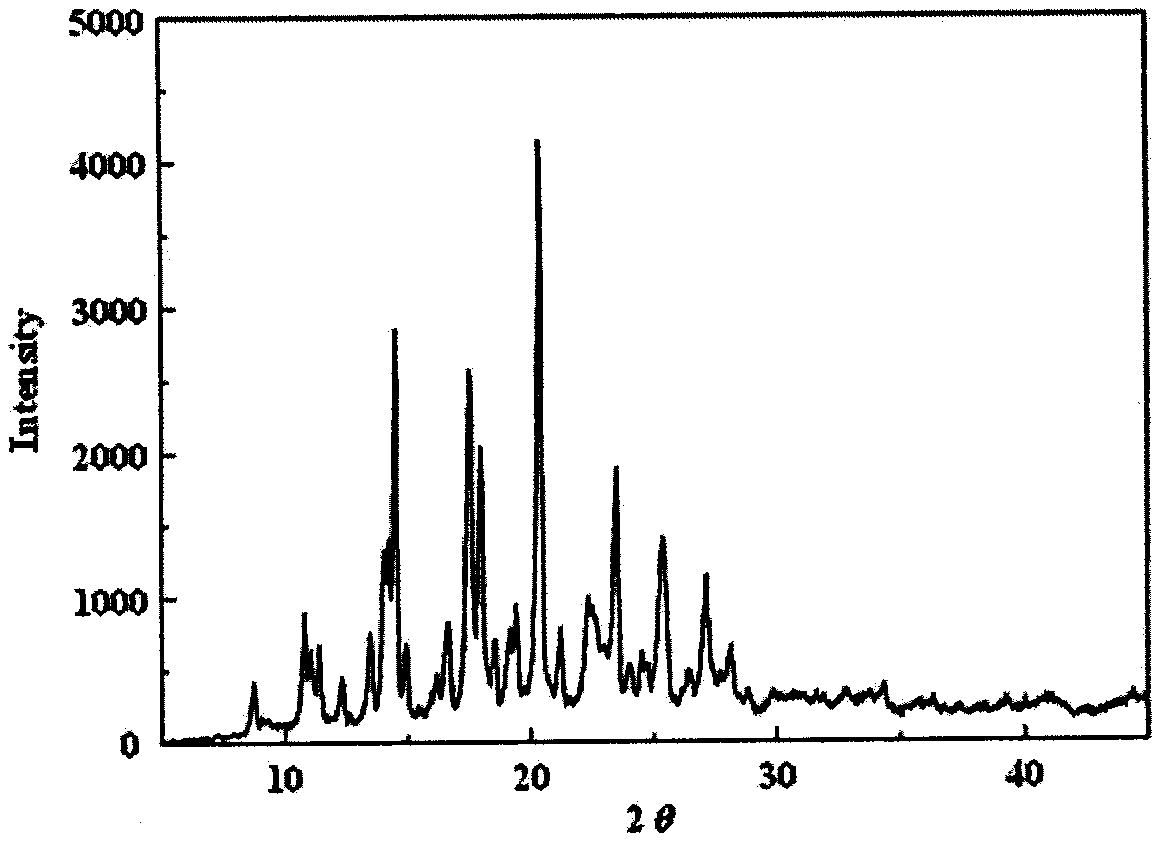
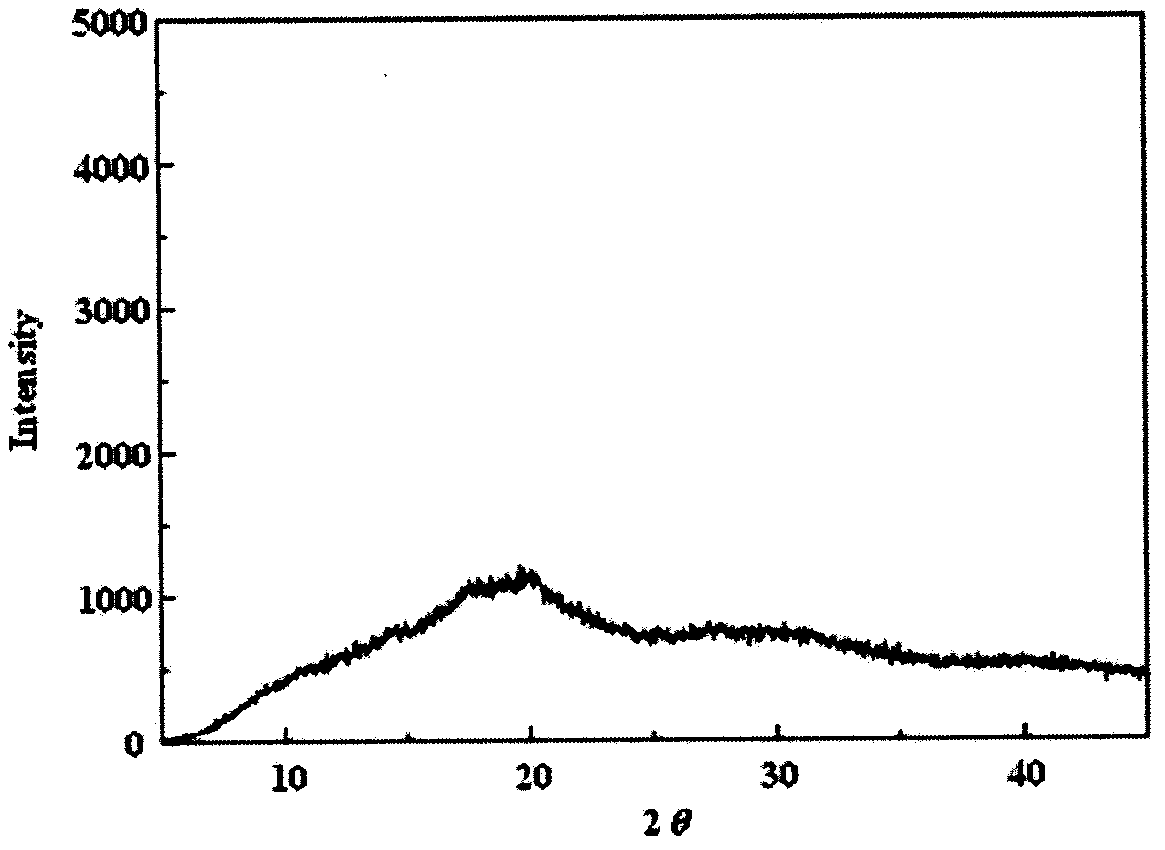
Examples
Embodiment 1
[0018] prescription:
[0019] Material composition
Prescription ratio (%)
Itraconazole
35
50
polyethylene glycol 6000
15
[0020] Preparation:
[0021] 1) Preparation of mixed solution: Accurately weigh the mixture of 1.75g itraconazole, 2.50g hypromellose and 0.75g polyethylene glycol 6000 into a 500mL conical flask, add 200mL ethanol and dichloromethane Mix solvents (volume ratio 1:2) to dissolve completely;
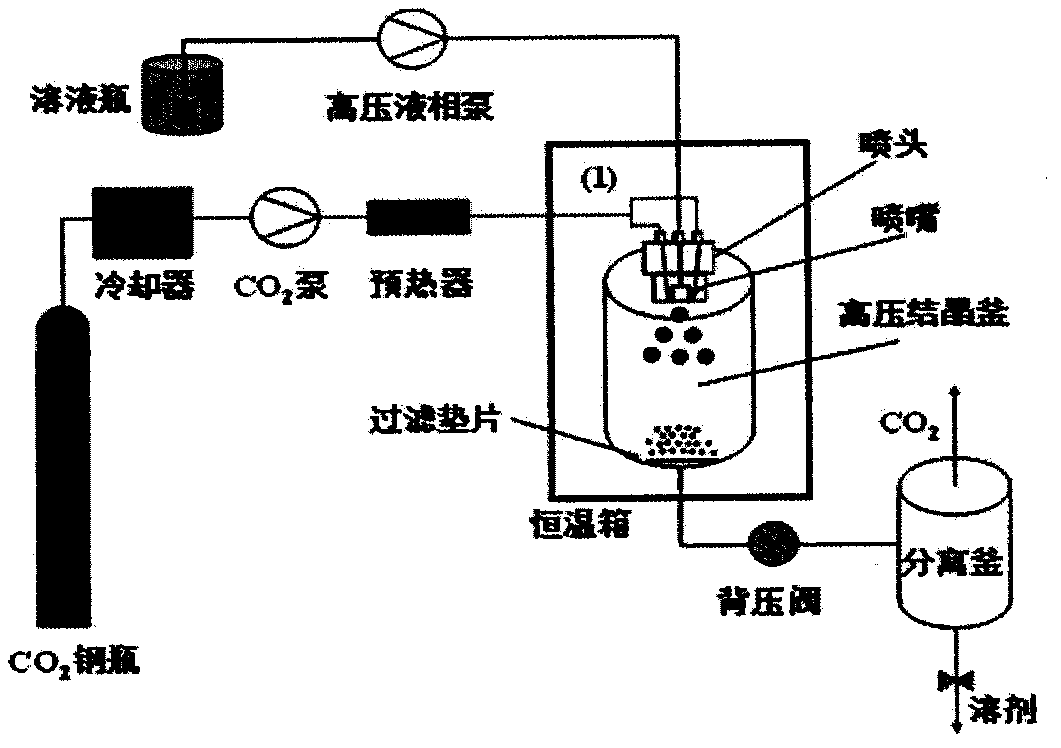
[0022] 2) CO2 feed: such as figure 1 As shown, open the valve of the CO2 cylinder, and the liquefied CO2 is continuously introduced into the high-pressure crystallization kettle of the supercritical fluid crystallization equipment system through the two symmetrical channels of the kettle head (60° from the middle solution channel) by the high-pressure pump, and the flow rate is controlled at 50mL / min;
[0023] 3) Control temperature and pressure: by regulating figure 1 The back pressure valve shown in t...
Embodiment 2
[0028] prescription:
[0029] Material composition
Prescription ratio (%)
Itraconazole
40
50
polyethylene glycol 6000
10
[0030] Preparation method: 1) Preparation of mixed solution: Accurately weigh the mixture of 1.6g itraconazole, 2.0g hypromellose and 0.4g polyethylene glycol 6000 into a 500mL conical flask, add 200mL ethanol and two A mixed solvent of methyl chloride (volume ratio 1: 1) to completely dissolve;
[0031] 2) CO 2 Feed: as figure 1 shown, open the CO 2 Cylinder valve, liquefied CO 2 The high-pressure pump is continuously introduced into the high-pressure crystallization kettle of the supercritical fluid crystallization equipment system through the two symmetrical channels of the kettle head (at 45° to the intermediate solution channel), and the flow rate is controlled at 75mL / min;
[0032] 3) Control temperature and pressure: by regulating figure 1 The back pressure valve shown controls the p...
Embodiment 3
[0037] prescription:
[0038] Material composition
Prescription ratio (%)
Itraconazole
40
hypromellose
40
polyethylene glycol 6000
20
[0039]Preparation method: 1) Preparation of mixed solution: Accurately weigh the mixture of 2.0g itraconazole, 2.0g hypromellose and 1.0g polyethylene glycol 6000 into a 500mL conical flask, add 200mL ethanol and two A mixed solvent of methyl chloride (volume ratio 1: 1) to completely dissolve;
[0040] 2) CO 2 Feed: as figure 1 shown, open the CO 2 Cylinder valve, liquefied CO 2 The high-pressure pump is continuously introduced into the high-pressure crystallization kettle of the supercritical fluid crystallization equipment system through the two symmetrical channels of the kettle head (60° to the intermediate solution channel), and the flow rate is controlled at 75mL / min;
[0041] 3) Control temperature and pressure: by regulating figure 1 The back pressure valve shown controls the press...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com