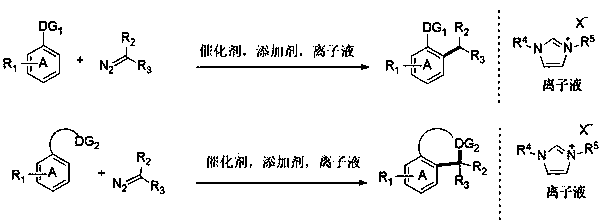
Novel green method of synthesizing C-C bond and N heterocycle derivatives through transition metal catalyzed C-H carbenoid coupling reaction
A technology of transition metal catalysis and coupling reaction, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, and bulk chemical production, etc. It can solve the problems of low atom utilization rate, inability to realize industrial production, and pollution Environmental and other issues, to achieve good thermal and chemical stability, improve atomic utilization, and improve the effect of reaction safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Implementation Example 1: Synthesis of Compound 1
[0032]
[0033] (1) Add 2-phenylpyridine (31.0 mg, 0.20 mmol), diethyl malonate diazo (44.7 mg, 0.24 mmol), dichloro(pentamethylcyclopentadiene base) rhodium(III) dimer (3.0 mg, 0.025 mmol), silver acetate (4.8 mg, 0.15 mmol), 1-butyl-3-methylimidazole bistrifluoromethanesulfonic acid imide salt (0.3 mL ), and stirred at room temperature for 2 hours.
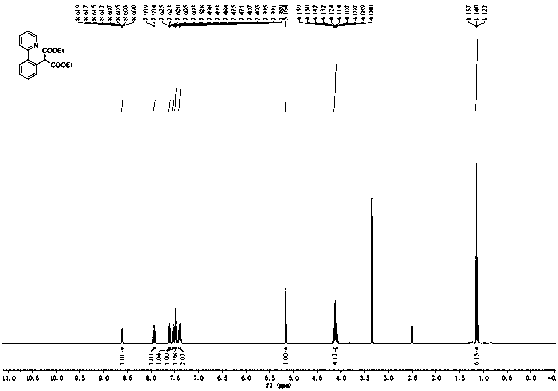
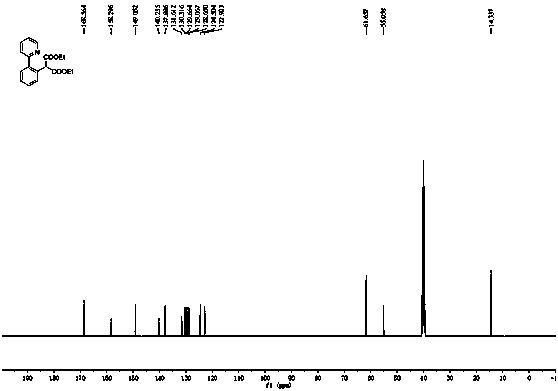
[0034] (2) After the reaction was completed, ether was added for extraction (1 mL×5), the ether layer was collected, the solvent was removed under reduced pressure, and the residue was separated by silica gel column chromatography (petroleum ether / ethyl acetate = 20 / 1, v / v) Purified to obtain 55 mg of the target product as a white solid with a yield of 95%; 1H NMR (400 MHz, DMSO- d 6 ) δ 8.61 (ddd, J = 4.8, 1.6, 0.8 Hz, 1H),7.94 (td, J = 7.6, 2.0 Hz, 1H), 7.61 (dt, J =7.6, 0.8 Hz, 1H), 7.56-7.52 (m,1H), 7.50-7.46 (m, 2H), 7.42-7.37 (m, 2H), 5.16 (s, 1H), 4.1...
Embodiment 2
[0035] Implementation Example 2: Synthesis of Compound 2
[0036]
[0037] (1) Add 3-trifluoromethylpyridine (44.6 mg, 0.20 mmol), diethyl malonate diazo (44.6 mg, 0.24 mmol), dichloro(pentamethylcyclopenta Dienyl) rhodium(III) dimer (3.1 mg, 0.025 mmol), silver acetate (5.0 mg, 0.15 mmol), 1-butyl-3-methylimidazole bistrifluoromethanesulfonate imide salt ( 0.3 mL), stirred at room temperature for 12 hours.
[0038] (2) After the reaction was completed, ether was added for extraction (1 mL×5), the ether layer was collected, the solvent was removed under reduced pressure, and the residue was separated and purified by silica gel column chromatography to obtain a colorless oily liquid with a yield of 81%; 1 H NMR (400 MHz, DMSO- d 6 ) δ 8.64(d, J = 4.4 Hz, 1H), 7.98 (t, J = 7.6 Hz, 1H), 7.89 (d, J = 8.4 Hz, 2H), 7.75(d, J = 7.6 Hz, 1H), 7.64 (d, J = 8.4 Hz, 1H), 7.49-7.44 (m, 1H), 5.21 (s,1H), 4.16-4.11 (m, 4H), 1.15 (t, J = 7.2 Hz, 6H); 13 C NMR (100 MHz, DMSO...
Embodiment 3
[0039] Implementation Example 3: Synthesis of Compound 3
[0040]
[0041] (1) In a clean reactor, add 2-(2-thiophene)pyridine (32.2 mg, 0.20 mmol), diethyl malonate diazo (44.7 mg, 0.24 mmol), dichloro(pentamethylcyclo Pentadienyl) rhodium(III) dimer (3.1 mg, 0.025 mmol), silver acetate (5.0 mg, 0.15 mmol), 1-butyl-3-methylimidazole bistriflate (0.3 mL), stirred at room temperature for 24 hours.
[0042] (2) After the reaction was completed, ether was added for extraction (1 mL×5), the ether layer was collected, the solvent was removed under reduced pressure, and the residue was separated and purified by silica gel column chromatography to obtain a yellow oily liquid with a yield of 95%; 1 H NMR (400 MHz, DMSO- d 6 ) δ 8.59(ddd, J = 4.8, 1.6, 0.8 Hz, 1H), 7.88 (td, J = 7.6, 1.6 Hz, 1H), 7.66-7.63(m, 2H), 7.34 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.10 (d, J = 4.8 Hz, 1H), 5.81(s, 1H), 4.15 (q, J = 7.2 Hz, 4H), 1.17 (t, J = 7.2 Hz, 6H); 13 C NMR (100 MHz, DMSO- ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com