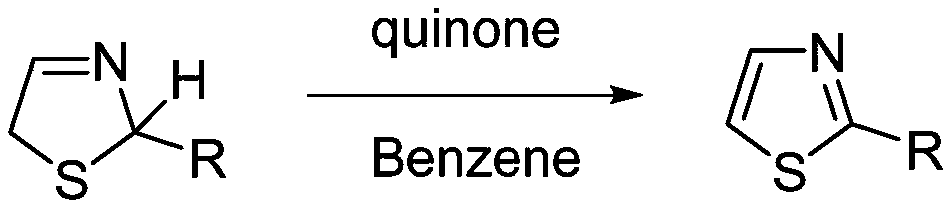
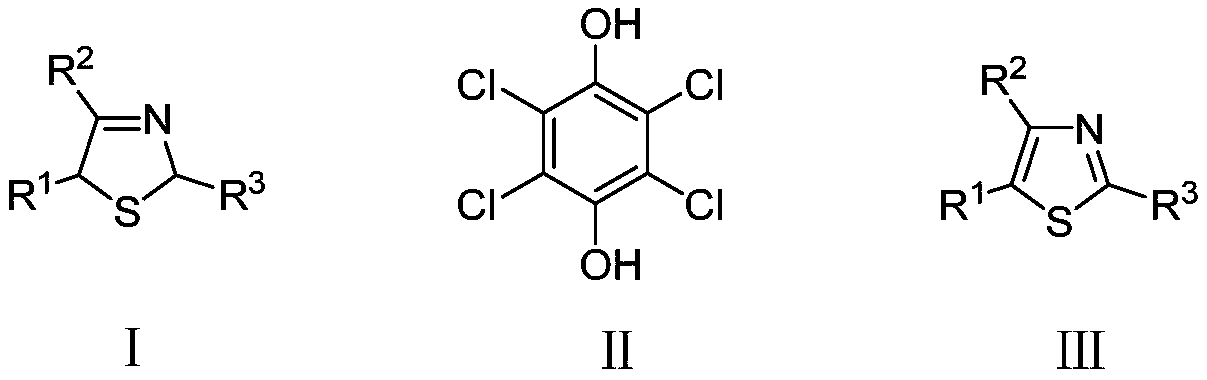
Electrochemical synthesis method of thiazole compounds
An electrochemical and thiazole technology, which is applied in the field of thiazole compound preparation, can solve the problems that the synthesis of thiazole compounds has not been reported in domestic and foreign literature, the post-processing process is complicated, and the environmental pollution is serious, and the cost is low, the equipment cost is low, and the experimental operation is simple. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Synthesis of 4,5-dimethyl-2-isobutylthiazole by electrochemical method
[0041] In a 50ml single-chamber electrolytic cell, add 4,5-dimethyl-2-isobutylthiazoline (1.0mmol), sodium bromide (1.0mmol), and tetrachlorohydroquinone (0.5mmol) to 5ml In the mixed two-phase solvent system of methyl chloride and 5ml water, graphite sheet electrode is used as anode, stainless steel mesh is used as cathode, and the temperature is 3mA / cm 2 Electrolysis under constant current, stirring at 40°C, stop electrolysis when the energization reaches 3.0F / mol, after cooling to room temperature, extract with dichloromethane, and wash with sodium hydroxide aqueous solution (0.5M) and water three times each, and pass through the column Chromatographic separation yielded 4,5-dimethyl-2-isobutylthiazole. Yield: 41%.
[0042]
[0043] Yellow liquid; 1 H NMR(400MHz, CDCl 3 )δ0.97(d,J=6.8Hz,6H),1.96-2.08(m,1H), 2.29(s, 3H), 2.30(s,3H), 2.75(d,J=7.2Hz,2H); 13 C NMR(100MHz, CDCl 3 )δ 11.2, 14.6...
Embodiment 2
[0044] Example 2: Synthesis of 4,5-dimethyl-2-isobutylthiazole by electrochemical method
[0045] In a 50ml single-chamber electrolytic cell, add 4,5-dimethyl-2-isobutylthiazoline (1.0mmol), sodium bromide (1.0mmol), and tetrachlorohydroquinone (0.5mmol) to 5ml 1 , 2-Dichloroethane and 5ml water mixed two-phase solvent system, graphite sheet electrode as anode, stainless steel mesh as cathode, at 3mA / cm 2 Electrolysis under constant current, stirring at 80°C, when the energization reaches 3.0F / mol, stop electrolysis, after cooling to room temperature, extract with dichloromethane, and wash with sodium hydroxide aqueous solution (0.5M) and water three times, and pass through column Chromatographic separation yielded 4,5-dimethyl-2-isobutylthiazole. Yield: 65%.
Embodiment 3
[0046] Example 3: Synthesis of 4,5-dimethyl-2-isobutylthiazole by electrochemical method
[0047] In a 50ml single-chamber electrolytic cell, add 4,5-dimethyl-2-isobutylthiazoline (1.0mmol), sodium bromide (1.0mmol), and tetrachlorohydroquinone (0.5mmol) to 5ml 1 ,2-Dichloroethane and 5ml water mixed two-phase solvent system, graphite electrode as anode, stainless steel mesh as cathode, at 3mA / cm 2 Electrolysis under constant current, stirring at 60℃, when the energization reaches 3.0F / mol, stop the electrolysis, after cooling to room temperature, extract with dichloromethane, and wash with sodium hydroxide aqueous solution (0.5M) and water three times, and pass through the column Chromatographic separation yielded 4,5-dimethyl-2-isobutylthiazole. Yield: 38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com