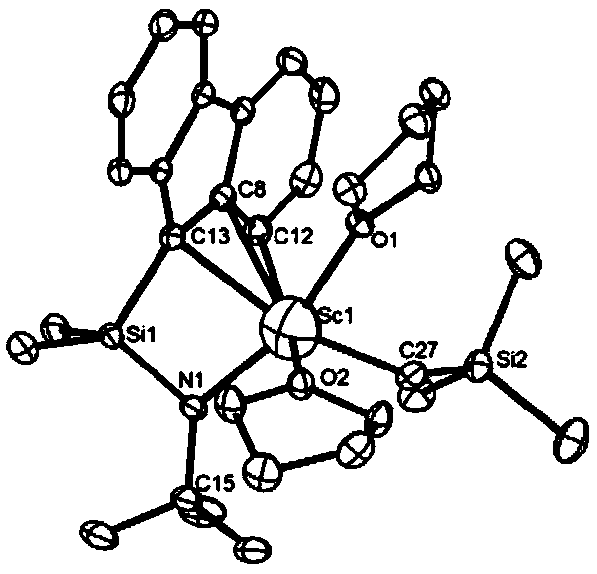
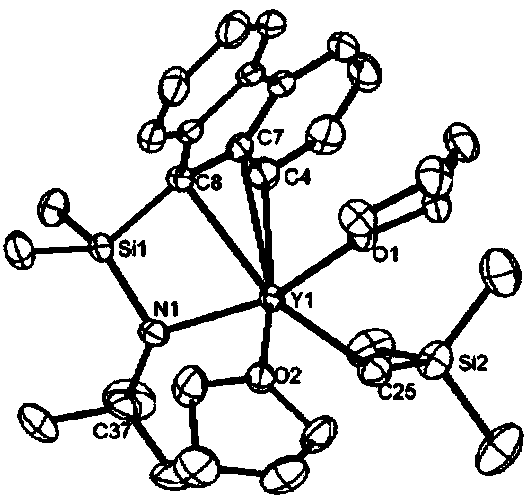
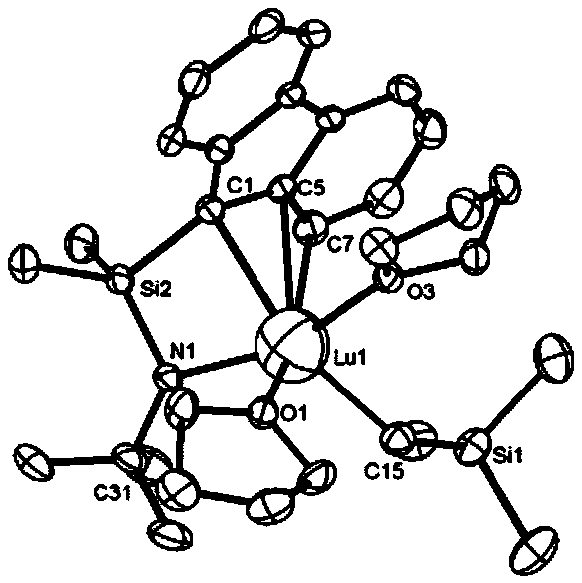
CGC type aminofluorene rare-earth metal catalyst and preparation method and application thereof
A technology of rare earth metals and catalysts, which is applied in the field of catalysts and can solve the problems that the application of preparation methods has not yet been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] (1) Preparation of aminofluorene ligand
[0078]
[0079] Step 1: First, add 8.35ml (20mmol, 2.4mol / L) of n-butyllithium dropwise to 3.33g (20mmol) of fluorene in tetrahydrofuran at -70°C under a nitrogen atmosphere to react for 2 hours to obtain mixture a; secondly , at -7°C, the mixture was added dropwise to the tetrahydrofuran solution of dichlorodimethylsilane and reacted for 5 hours to obtain the mixture b; finally, the reaction solution was drained, extracted three times with n-hexane, and the extract was drained to obtain The product of the first step is 3.188g, and the yield is 61.3%.
[0080] The second step: under the protection of nitrogen at room temperature, 0.8 g (10 mmol) of tert-butylamine and 4 g (4 mmol) of triethylamine were respectively added to the tetrahydrofuran solution of the first step product 2.6 g (10 mmol) and stirred for 5 h; the reaction solution was drained, After extraction with n-hexane, the extract was concentrated and transferred ...
Embodiment 2
[0085] Preparation of CGC-type Aminofluorene Lutetium Catalyst
[0086]
[0087] First, in the glove box, 591 mg (2 mmol) of the aminofluorene ligand was added to a round-bottomed flask, and 10 ml of n-hexane was used as a solvent to fully dissolve; secondly, Lu(CH 2 SiMe 3 ) 3 thf 2 1.16g (2mmol) was dissolved in 3mL of n-hexane, and the n-hexane solution in which the aminofluorene ligand was dissolved was added dropwise to the n-hexane solution in which the metal source was dissolved, and stirred at room temperature for 3 hours, a yellow solid precipitated; finally, centrifuged to obtain a yellow The solid was washed three times with cold n-hexane to obtain 1.08 g of aminofluorene catalyst with a yield of 75.4%.
Embodiment 3
[0089] Preparation of CGC type amine fluorene yttrium catalyst
[0090]
[0091] First, in the glove box, 591 mg (2 mmol) of the aminofluorene ligand was added to a round-bottomed flask, and 10 ml of n-hexane was used as a solvent to fully dissolve it; secondly, Y(CH 2 SiMe 3 ) 3 thf 2 1g (2mmol) was dissolved in 3mL of n-hexane, and the n-hexane solution in which the aminofluorene ligand was dissolved was added dropwise to the n-hexane solution in which the metal source was dissolved, and stirred at room temperature for 3 hours, a yellow solid precipitated; finally, centrifuged to obtain a yellow solid , washed three times with cold n-hexane to obtain 1.09 g of aminofluorene catalyst with a yield of 85.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com