Estrone derivative, immunogen, antibody, enzyme-labeled conjugate, detection reagent and preparation method thereof
A technology of derivatives and immunogens, applied in the fields of enzyme-labeled conjugates, detection reagents and their preparation, immunogens, antibodies, and estrone derivatives, which can solve problems such as errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
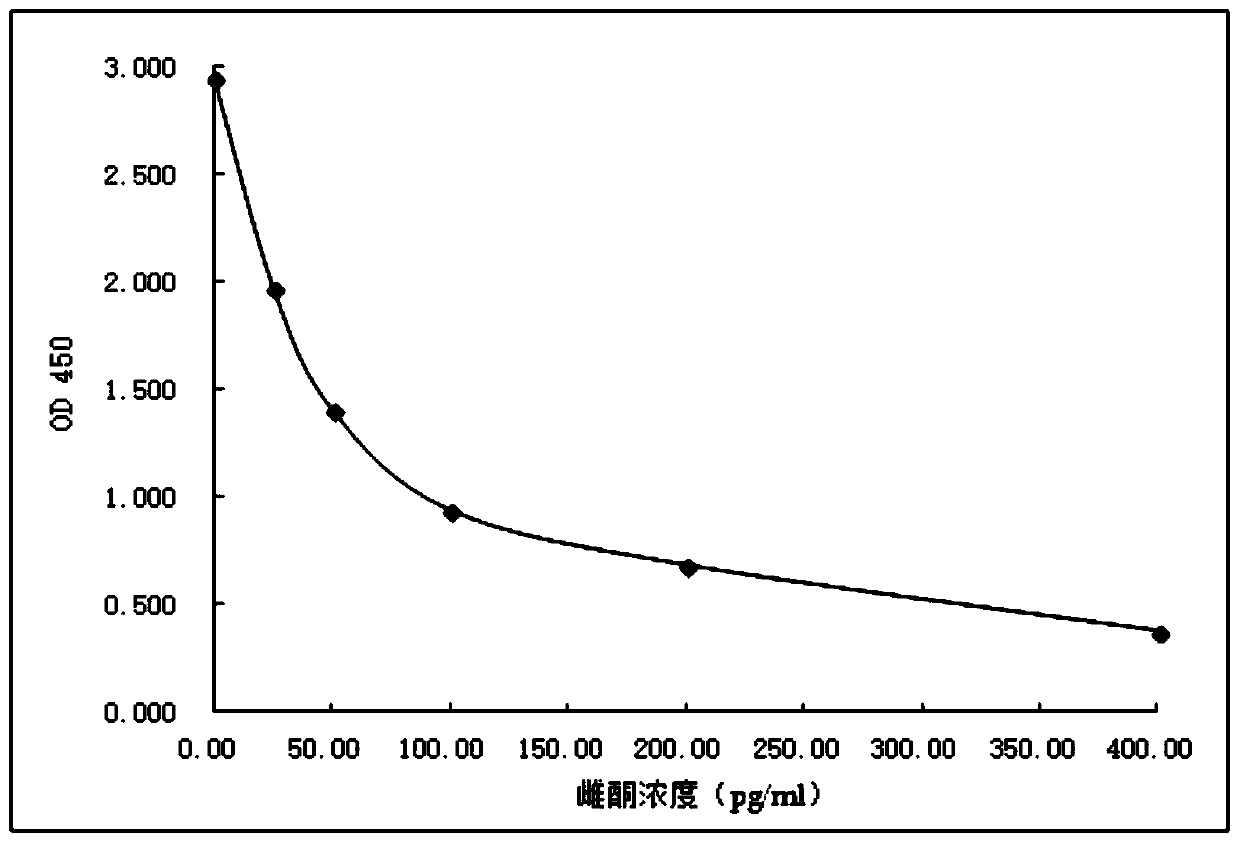
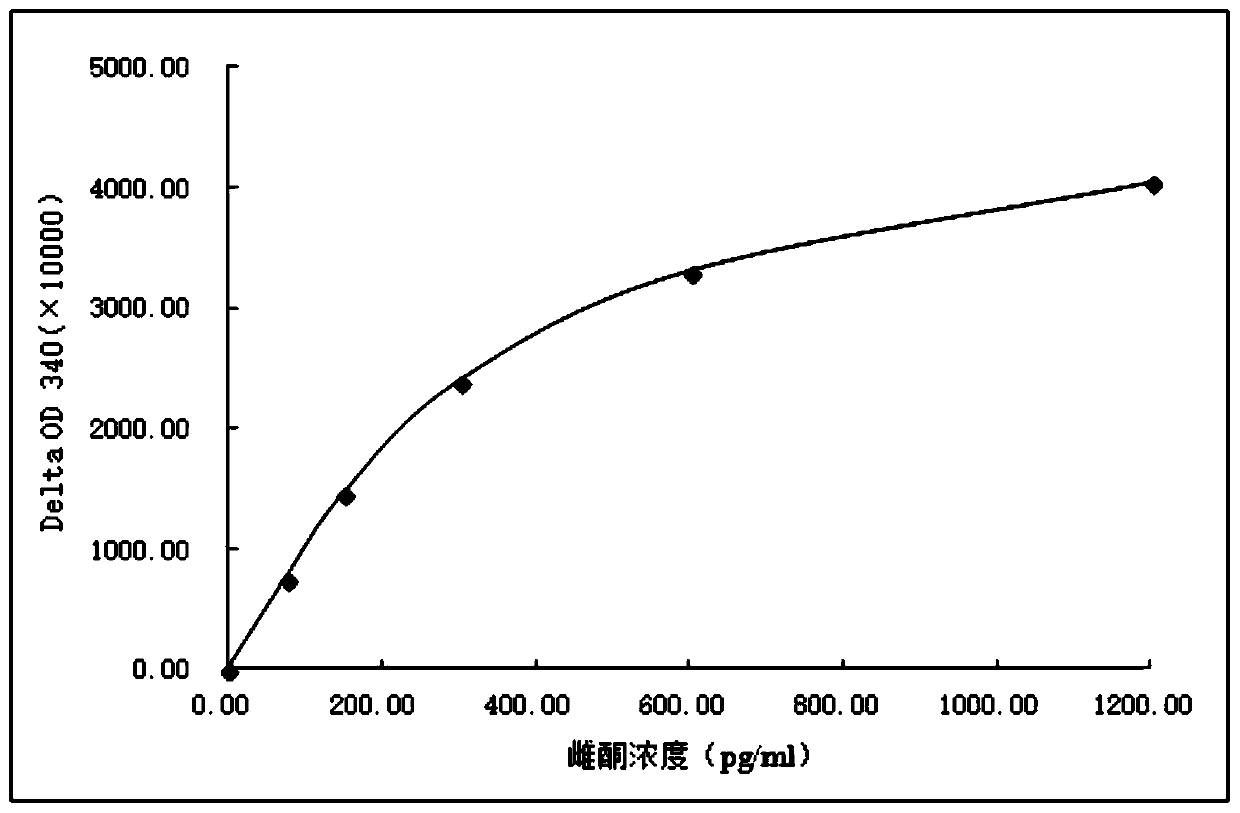
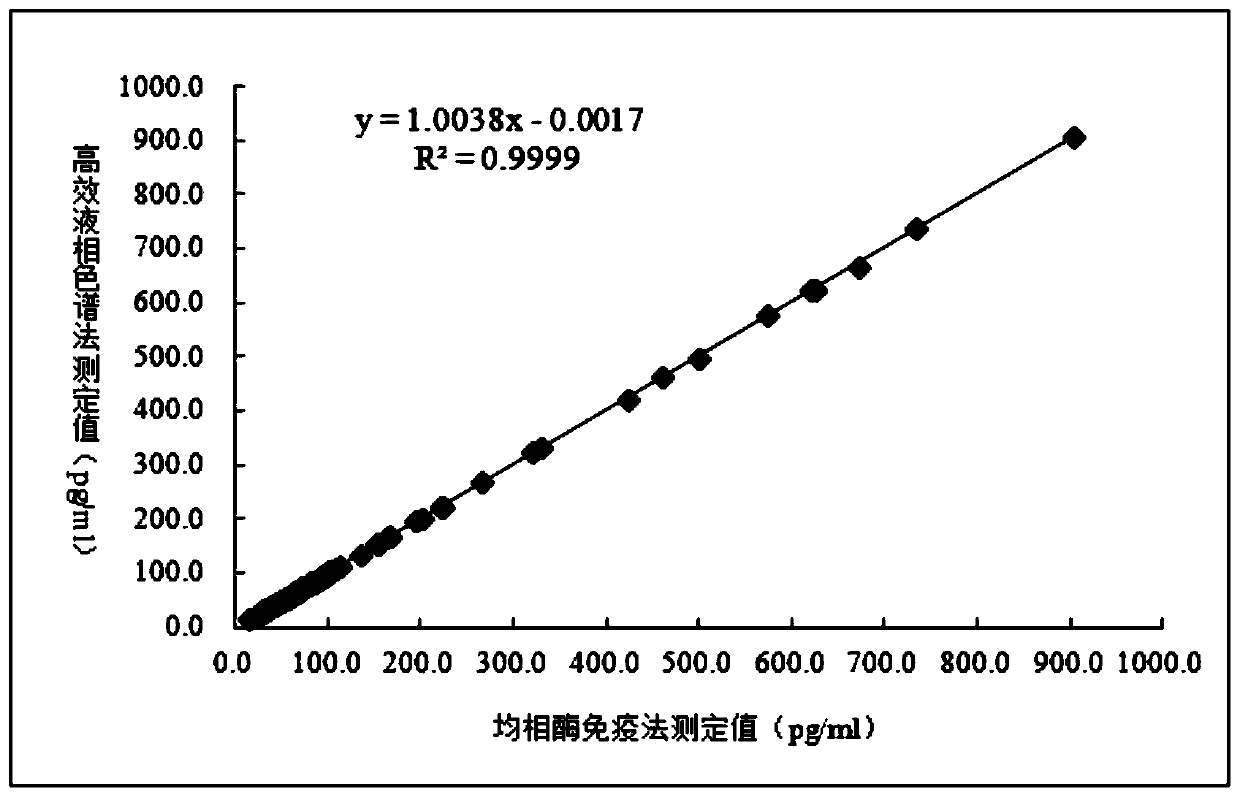
Image
Examples
Embodiment 1
[0080] Embodiment 1. Synthesis of estrone derivatives
[0081] The chemical structure of estrone derivatives is shown in formula (I):
[0082]
[0083] The synthetic route and preparation steps of the above-mentioned estrone derivatives are as follows:
[0084]
[0085] Concrete synthetic steps are as follows:
[0086] (1) Synthesis of compound 3
[0087]
[0088] Weigh 5.0g (0.018mol) of compound 1 and 10g (0.048mol) of compound 2 and dissolve in 100mL of acetone, then add 7.9g (0.057mol) of K 2 CO 3 , to prepare a reaction mixture solution, and then the reaction mixture solution was heated to reflux for 12 hours. The solution after the reaction was completed was filtered, and the residue obtained after removing the solvent was purified through a silica gel purification column to obtain 3.0 g of compound 3 as a white solid.
[0089] (2) Synthesis of estrone derivatives
[0090]
[0091] Weigh 3.0g (7.54mmol) of compound 3, 1.1g (11.3mmol) of compound 4, 2.96...
Embodiment 2
[0093] Example 2. Synthesis of Estrone Immunogen
[0094] The estrone immunogen in this example is formed by linking the estrone derivative shown in the formula (I) of Example 1 with bovine serum albumin (BSA), and its structural formula is shown in the following formula (II):
[0095]
[0096] The carrier is bovine serum albumin.
[0097] The concrete steps of the synthetic method of this estrone immunogen are as follows:
[0098] (1) Weigh 2.72g of potassium dihydrogen phosphate, 4.26g of disodium hydrogen phosphate, 8.5g of sodium chloride, and 0.95g of magnesium chloride, dissolve them in 1L of deionized water, adjust the pH to 8.2, and make buffer solution A;
[0099] (2) Weigh 3mg of BSA and dissolve it in 3mL of the above buffer solution A at -4°C to make a 1mg / ml carrier solution;
[0100] (3) Weigh 3 mg of the estrone derivative represented by the above formula (I), and dissolve it in 300 μl of the above buffer solution A at -4°C to prepare a 10 mg / ml estrone der...
Embodiment 3
[0103] Synthesis of Example 3 Estrone Immunogen
[0104] The estrone immunogen in this example is formed by linking the estrone derivative shown in the formula (I) of Example 1 with bovine serum albumin (BSA), and its structural formula is shown in the following formula (II):
[0105]
[0106] The carrier is bovine serum albumin.
[0107] The concrete steps of the synthetic method of this estrone immunogen are as follows:
[0108] (1) Weigh 2.3g of potassium dihydrogen phosphate, 3.8g of disodium hydrogen phosphate, 8.0g of sodium chloride, and 0.7g of magnesium chloride, dissolve them in 0.9L of deionized water, adjust the pH to 8.0, and make buffer solution A;
[0109] (2) Weigh 6mg of BSA and dissolve it in 3mL of the above buffer solution A at -4°C to make a 2mg / ml carrier solution;
[0110] (3) Weigh 2.4 mg of the estrone derivative represented by the above formula (I), and dissolve it in 300 μl of the above buffer solution A at -4°C to prepare an 8 mg / ml estrone deriv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com