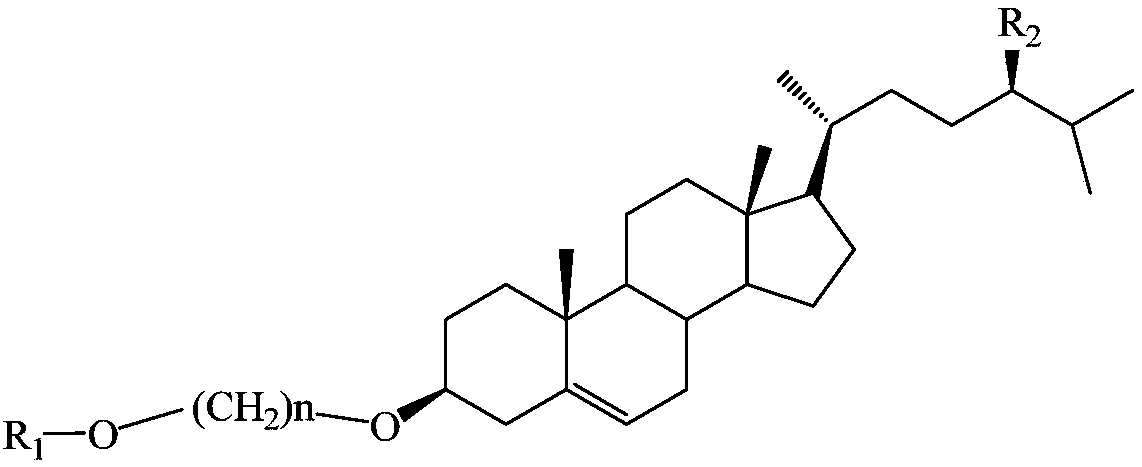
Small-molecule gel, method for preparing same and gynecological solid-liquid mutual transformation type gel preparation
A molecular gel and gel preparation technology, applied in the field of gynecological pharmacy, can solve the problems of unsatisfactory stability of small molecule gel, uneven drug distribution, poor stability of raw materials, etc., and achieves good application prospects, solid- Strong liquid interconversion ability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
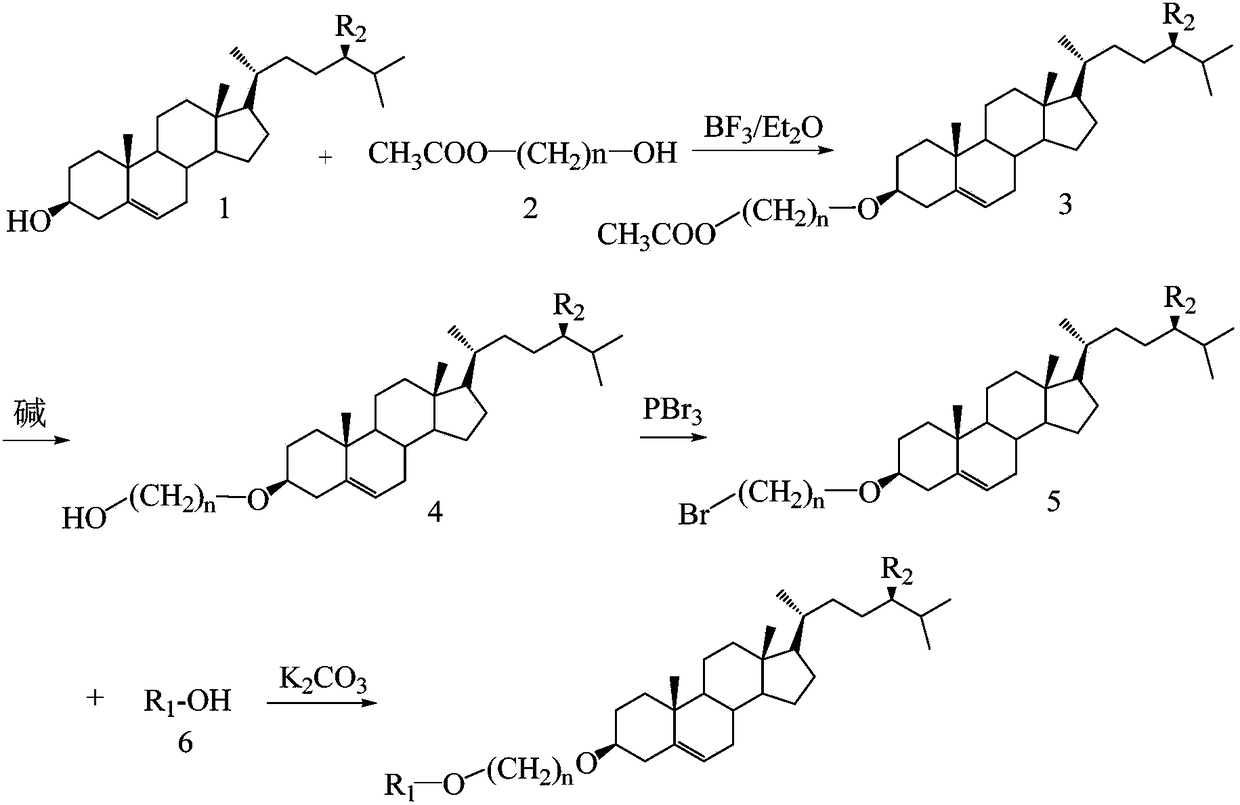
Examples
Embodiment 1
[0029]
[0030] 1. Etherification reaction
[0031] Add 100 mL of ether, 19.32 g (0.05 mol) of cholesterol (compound 1-1), 6.49 g (0.055 mol) of propylene glycol monoacetate (compound 2-1), and 7.63 mL of tris The ether solution of boron fluoride (0.025mol) was refluxed at 34°C for 8 hours. After the reaction, 100mL of water was added to recover the ether, and petroleum ether was used as the eluent. The product was separated and purified by silica gel column chromatography to obtain 19.46g of compound 3 -1, yield 80%.
[0032] 2. Hydrolysis reaction
[0033] Add 100 mL of ethanol, 14.59 g (0.03 mol) of compound 3-1, 1.32 g (0.033 mol) of NaOH into a round bottom flask, reflux at 78°C for 4 hours, add 100 mL of water after the reaction, recover ether, and obtain 12.67 g of compound 4 -1, yield 95%.
[0034] 3. Bromination reaction
[0035] Add 50 mL of ether, 8.88 g (0.02 mol) of compound 4-1, 2.71 g (0.01 mol) of PBr into the round bottom flask 3 , reflux reaction at 3...
Embodiment 2
[0039] In step 4 of this example, the 7-hydroxycoumarin in step 4 of example 1 is replaced with equimolar 6-hydroxyflavone, and the other steps are the same as in example 1 to obtain 6.23 g of the small compound shown in formula 7-2. Molecular gelling agent, the yield is 90%, and the total yield is 65%.
[0040]
[0041] The structural characterization data of the obtained small molecule gel is: 1 H NMR (CDCl 3 ,TMS):8.11-8.09(2H,d,J=6.11Hz),7.69(1H,d,J=8.05Hz),7.65(1H,s),7.61(3H,m),7.37-7.39(1H, d,J=3.05Hz),7.30-7.27(1H,dd,J=8.05Hz,J=3.05Hz),5.37(1H,s),3.88-3.87(2H,t),3.50(3H,m), 2.27-2.28(2H,m),2.07-2.08(2H,m),0.67-1.85(41H); 13C NMR (CDCl 3 ):176.9,162.1,154.8,149.3,140.8,131.5,131.3,129.1,128.8,126.1,124.2,123.0,120.0,107.4,105.8,72.0,65.1,56.4,42.7,40.1,331.5,361.3,37. 28.0, 24.4, 22.8, 21.0, 19.1, 18.5, 11.2.
Embodiment 3
[0043] In step 1 of this example, the cholesterol in step 1 of example 2 is replaced with equimolar β-sitosterol, and the other steps are the same as in example 2 to obtain the small molecule gelling agent shown in formula 7-3, with a total yield of 64%.
[0044]
[0045] The structural characterization data of the obtained small molecule gel is: 1 H NMR (CDCl 3 ,TMS):8.11-8.09(2H,d,J=6.11Hz),7.69(1H,d,J=8.05Hz),7.65(1H,s),7.61(3H,m),7.37-7.39(1H, d,J=3.05Hz),7.30-7.27(1H,dd,J=8.05Hz,J=3.05Hz),5.37(1H,s),3.88-3.87(2H,t),3.50(3H,m), 2.27-2.28(2H,m),2.07-2.08(2H,m),0.67-1.85(45H); 13 C NMR (CDCl 3 ):176.9,162.1,154.8,149.3,140.8,131.5,131.3,129.1,128.8,126.1,124.2,123.0,120.0,107.4,105.8,72.0,65.1,56.4,46.0,42.7,40,136.3,37. 28.0, 24.4, 23.4, 22.8, 21.0, 19.1, 18.5, 12.1, 11.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com