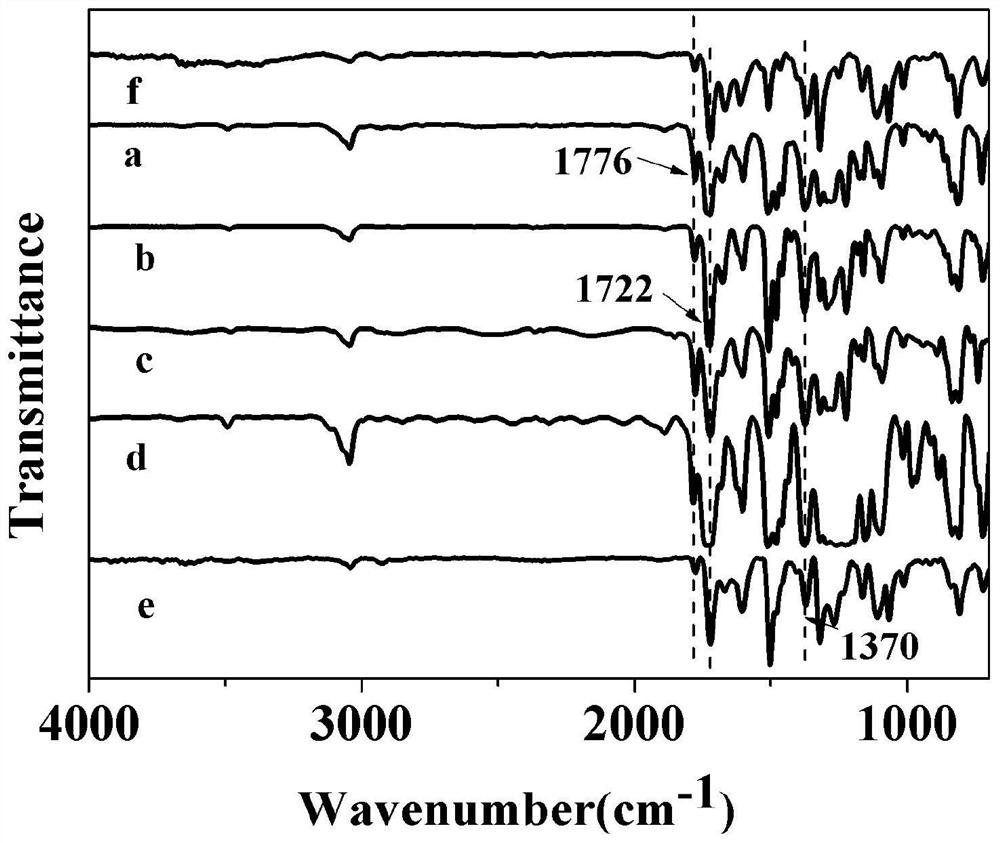
A kind of hyperbranched polyimide containing phenanthrene ring structure and its preparation method and application
A technology of polyimide and polyimide film, which is applied in the field of material science, can solve problems such as strong interaction, insolubility and infusibility, and achieve the effects of low condition requirements, improved thermal performance, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
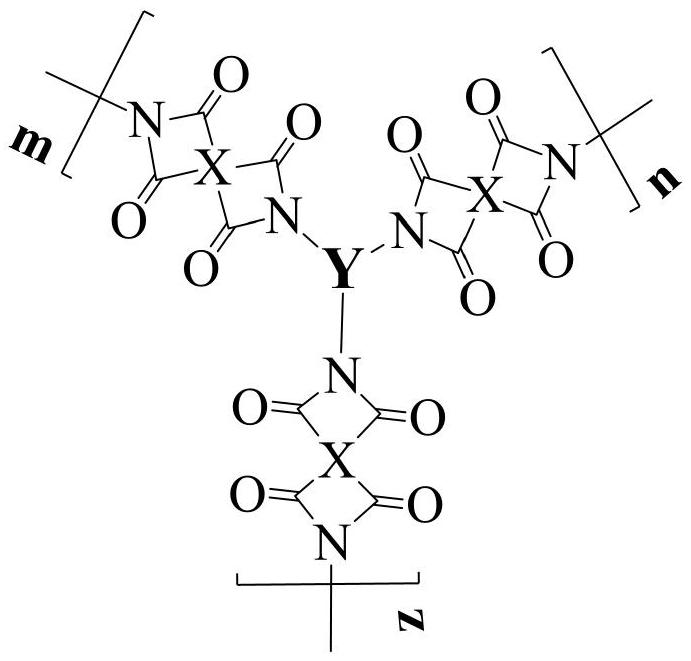
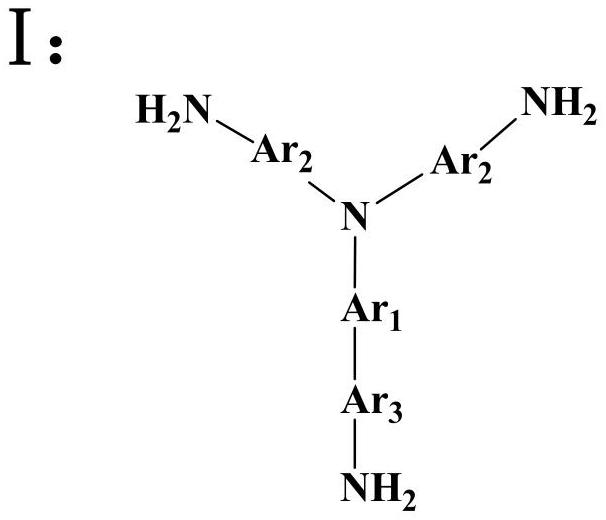
[0037] Add 0.4362g (2mmol) of pyromellitic dianhydride (PMDA) and 36ml of N,N-dimethylformamide into a three-necked flask, blow in argon, raise the temperature to 30°C, and add the triamine monomer N 2 -(6-aminonaphthalen-2-yl)-N 2 -(10-(6-aminonaphthalen-2-yl)phenanthren-2-yl)naphthalene-2,6-diamine 0.6168g (1mmol) was dissolved in 40ml N,N-dimethylformamide with constant pressure dropping funnel in 1~2h, evenly drop into the three-necked flask, then continue to react for 14h, then add 6ml of acetic anhydride and 2ml of triethylamine, heat up to 45°C and continue to react for 12h, after the reaction is completed, cool to room temperature and discharge in ethanol, filter, wash, Repeat 2 to 3 times, and finally place it in a vacuum drying oven at 80°C for 24 hours to obtain a dark reddish-brown hyperbranched polyimide polymer, whose structural formula is as follows:
[0038]
Embodiment 2
[0040] Add 0.4515g (2.07mmol) of pyromellitic dianhydride (PMDA) and 15ml of N,N-dimethylacetamide into a three-necked flask, blow in argon, raise the temperature to 30°C, and add the triamine monomer N 1 -(4-aminophenyl)-N 1 -(1-(4-aminophenyl)phenanthren-4-yl)benzene-1,4-diamine 0.4666g (1mmol) dissolved in 15ml N,N-dimethylacetamide with a constant pressure dropping funnel in 1 ~ 2h Add it dropwise into a three-necked flask, then continue to react for 16 hours, then add 6.2ml of acetic anhydride and 2.1ml of triethylamine, heat up to 45°C and continue to react for 15 hours, after the reaction is completed, cool to room temperature and discharge in methanol, filter, wash, repeat 2 ~3 times, and finally placed in a vacuum drying oven at 80°C for 24 hours to obtain a yellow hyperbranched polyimide polymer, whose structural formula is as follows:
[0041]
Embodiment 3
[0043]Add 0.4413g (1.5mmol) of 3,3',4,4'--biphenyltetracarboxylic dianhydride (BPDA) and 10ml of N-methylpyrrolidone into a three-necked flask, pass in argon, raise the temperature to 30°C, and Triamine monomer 7,7'-((3-(7-amino-9-oxo-9H-fluoren-2-yl)phenanthren-9-yl)azanediyl)bis(2-amino-9H-fluoren-9- one) 0.7729g (1mmol) was dissolved in 8ml of N-methylpyrrolidone with a constant pressure dropping funnel and evenly added dropwise into the three-necked flask for 1 to 2 hours, then continued to react for 24 hours, then added 12ml of acetic anhydride and 3ml of triethylamine, and raised the temperature to Continue to react at 45°C for 15h. After the reaction is completed, cool to room temperature and discharge the material in ethanol, filter, wash, repeat 2-3 times, and finally place it in a vacuum oven at 80°C for 24h to obtain a brown hyperbranched polyimide polymer. substance, its structural formula is as follows:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com