Trialkoxysilanes connected with thioether bond on alpha-carbon and containing different functional groups based on sulfydryl-ene click reaction, and preparation
A technology of chloromethyltrialkoxysilane and mercaptomethyltrialkoxysilane, which is applied in the field of organosilicon synthesis, can solve the problems of unsatisfactory appearance, stability and purity, and reduce the use and reaction of toxic catalysts. The effect of mild conditions and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
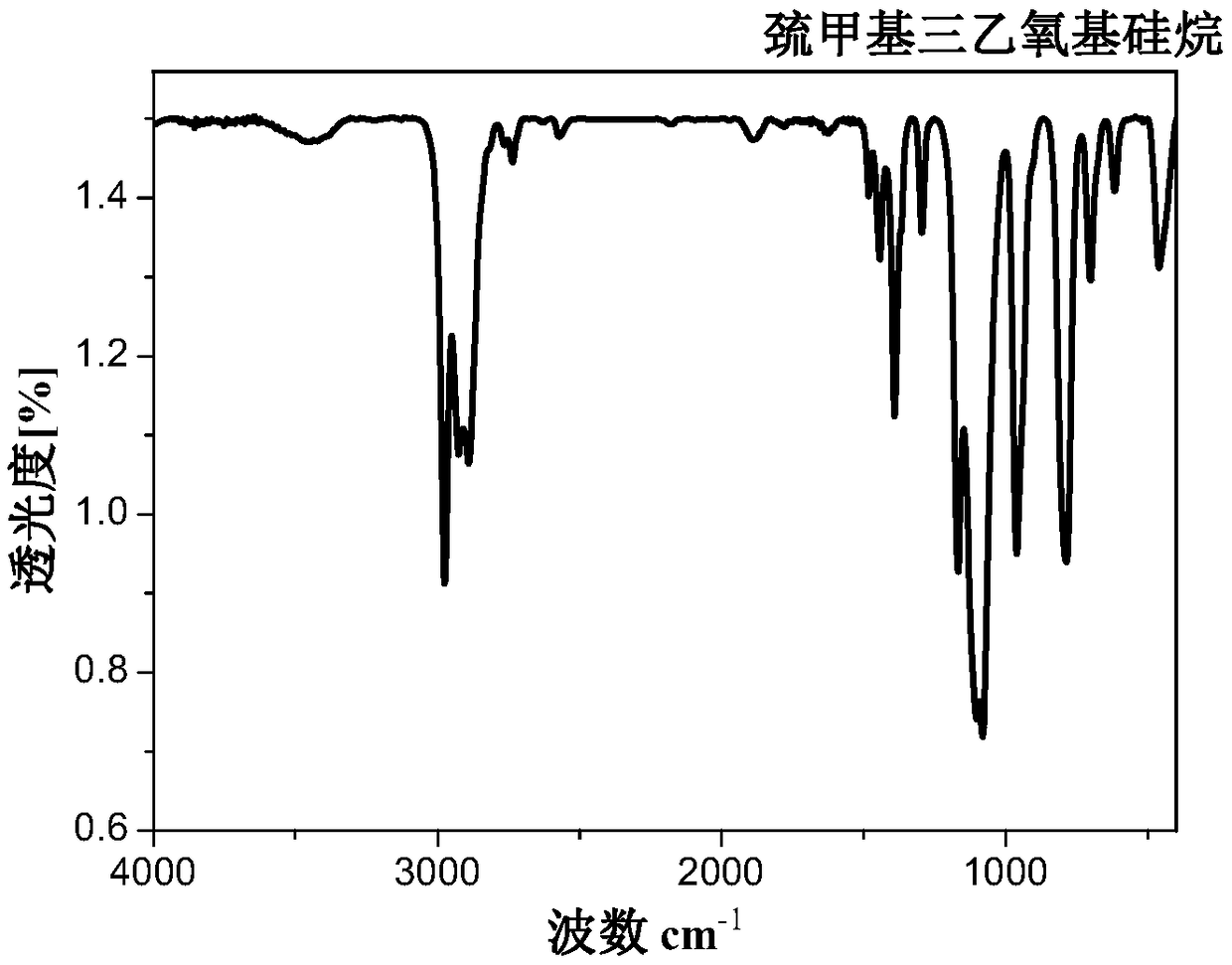
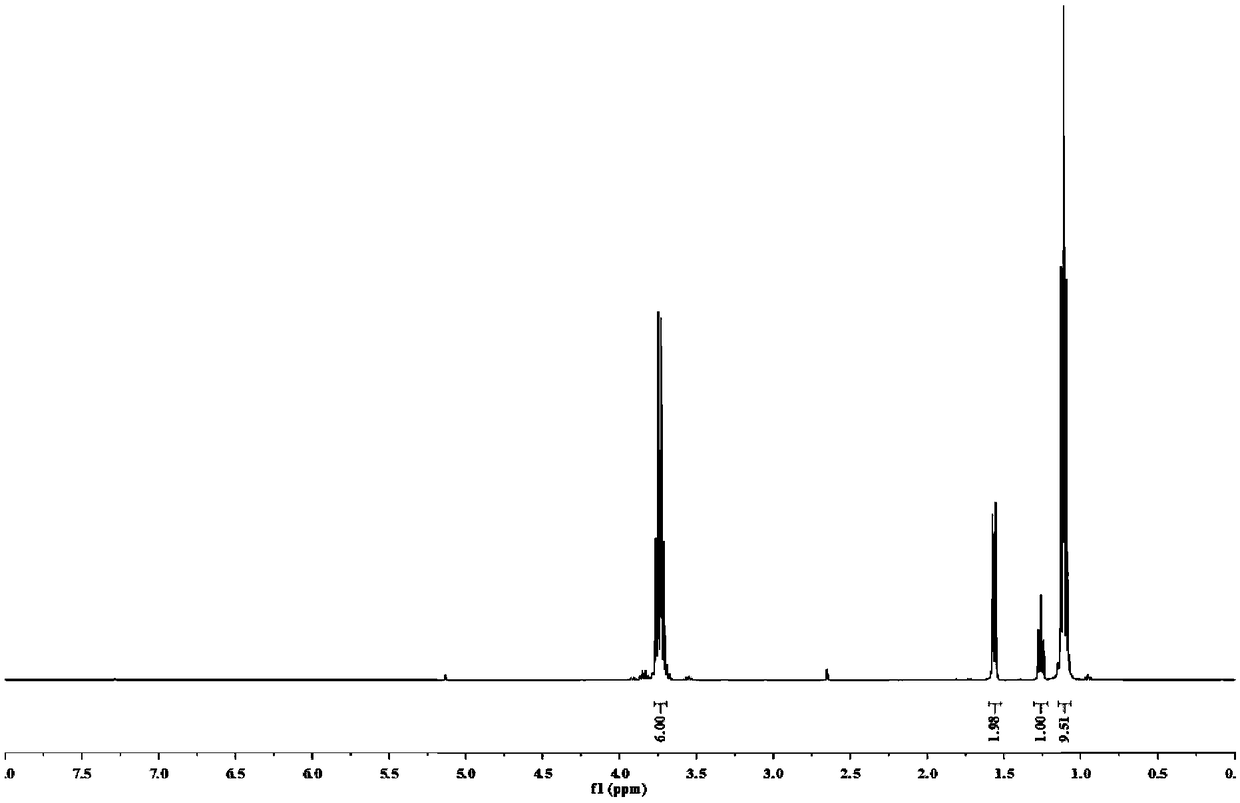
[0077] A preparation method of α-mercaptomethyltriethoxysilane, comprising steps as follows:
[0078] (1) Add 19 mL of chloromethyltrichlorosilane to a 250 mL dry three-necked flask, dissolve 31 g of sodium ethoxide in 110 mL of ethanol solvent, and slowly drop it into the three-necked flask through a constant pressure dropping funnel under nitrogen protection , the rate of addition was 1 drop / second, and the addition was stopped when the pH of the system changed from acidic to neutral. After reacting at room temperature for 1 h, the organic solvent was removed by distillation under normal pressure and the insoluble matter was filtered off, and then distilled under reduced pressure to obtain chloromethyltriethoxysilane, which was reserved for later use.
[0079] (2) Add 63.105g of chloromethyltriethoxysilane, 25.120g of thiourea and 0.957g of potassium iodide into a 250mL dry three-necked round-bottomed flask, heat to 110°C, keep the reaction for 1.5h in a nitrogen atmosphere,...
Embodiment 2
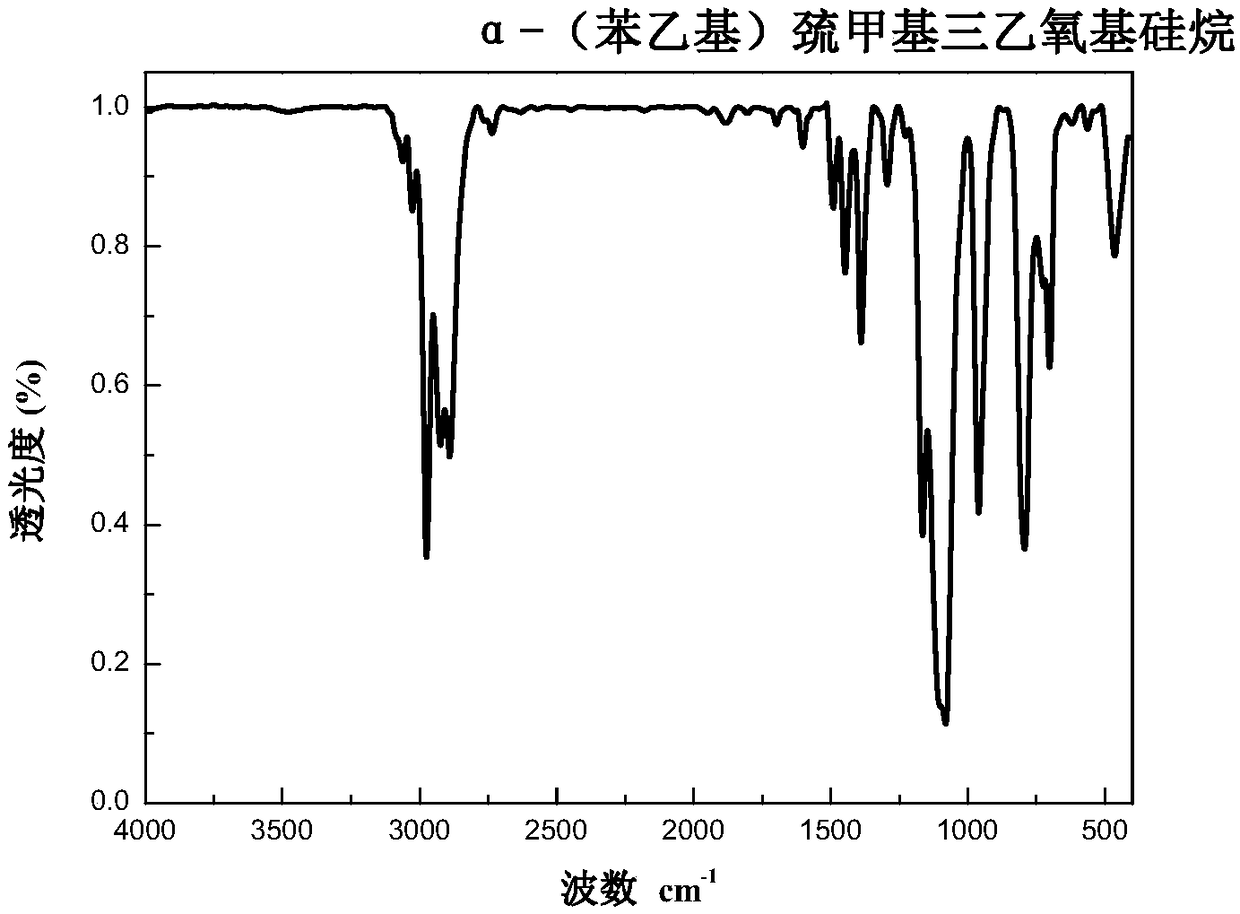
[0082] Preparation of α-(Phenylethyl)mercaptomethyltriethoxysilane
[0083] The structural formula of α-(phenethyl)mercaptomethyltriethoxysilane is as formula (III):
[0084]
[0085] Including the following steps:
[0086] Add 2.630 g of α-mercaptomethyltriethoxysilane prepared in Example 1 and 1.302 g of styrene into a round-bottomed flask wrapped in aluminum foil, then add 8 mL of tetrahydrofuran, mix well, then add 0.079 g of benzoin dimethyl ether, Stir evenly, remove the aluminum foil, and irradiate for 30 minutes at room temperature under a UV lamp with a power of 20W and a wavelength of 365nm. After the reaction, the solvent was distilled off under reduced pressure, the vacuum degree was 5-6mmHg, precipitated in n-hexane three times, and dried in vacuum at 40°C to constant weight to obtain α-(phenylethyl)mercaptomethyltriethoxysilane , with a purity of 97% and a yield of 96%.
[0087] The infrared spectrum of the product α-(phenylethyl)mercaptomethyltriethoxysila...
Embodiment 3
[0089] Preparation of α-(methyl propionate)mercaptomethyltriethoxysilane
[0090] The structural formula of α-(methyl propionate) mercaptomethyltriethoxysilane is as formula (IV):
[0091]
[0092] Including the following steps:
[0093]Add 2.640g of α-mercaptomethyltriethoxysilane prepared in Example 1 and 1.290g of methyl acrylate into a round-bottomed flask wrapped in aluminum foil, then add 8mL of tetrahydrofuran, mix well and then add 0.079g of benzoin dimethyl ether , stir evenly, remove the aluminum foil, and irradiate for 30 minutes at room temperature under a UV lamp with a power of 20W and a wavelength of 365nm. After the reaction, the solvent was distilled off under reduced pressure, the vacuum degree was 5-6mmHg, precipitated in n-hexane three times, and dried in vacuum at 40°C to constant weight to obtain α-(methylpropionate)mercaptomethyltriethoxy silane with a purity of 97% and a yield of 96%.
[0094] The infrared spectrogram of the product α-(methyl prop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com