A kind of preparation method of naphthalene-1,8-diaminoarylboronamide
A technology of diaminoarylboronamide and dibenzoyl peroxide, which is applied in the field of compound preparation, can solve the problems of reduced reaction efficiency, complicated post-treatment, low yield, etc., and achieves high reaction conversion rate, low price, good tolerance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
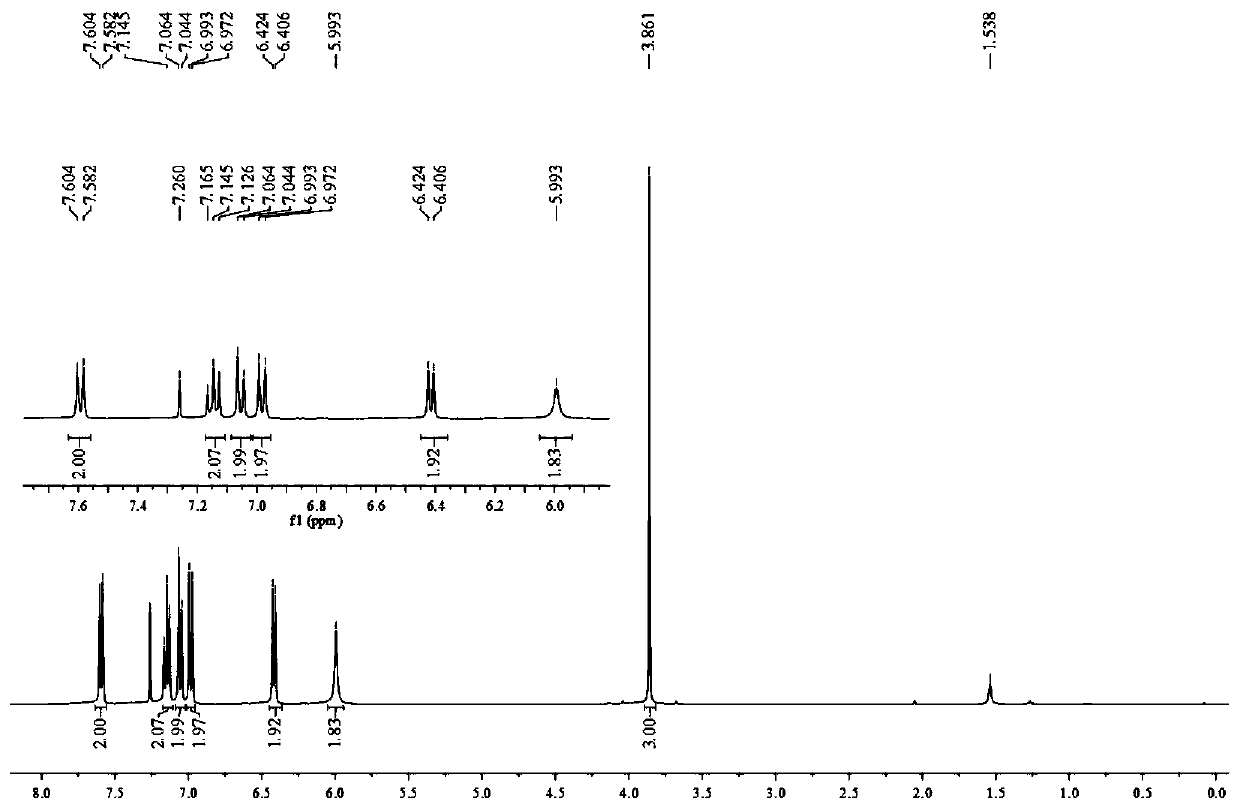
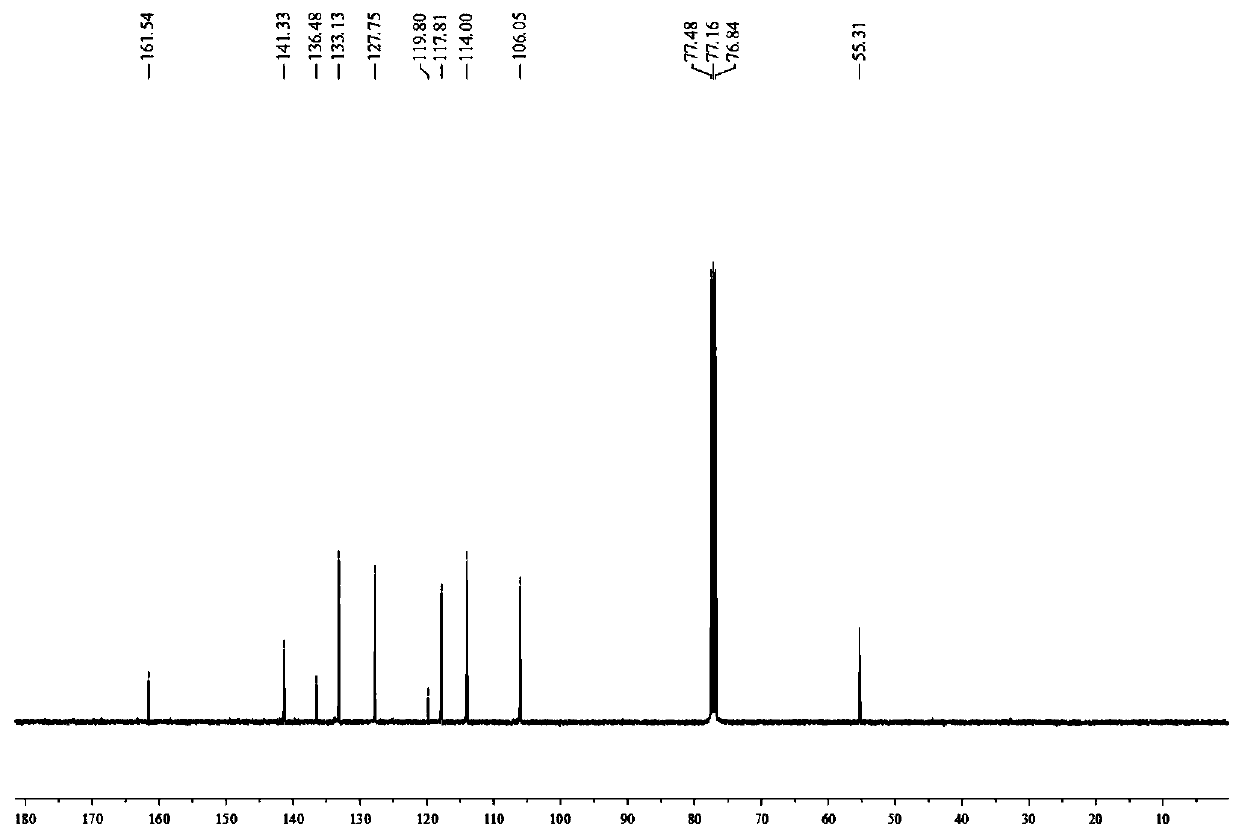
[0033] Add 4-methoxyaniline (24.6mg, 0.2mmol, 2.0eq.), Bpin-Bdan (29.4mg, 0.1mmol, 1.0eq.), tetrabutylammonium iodide (3.7mg , 0.01mmol, 0.1eq.), sodium acetate (12.3mg, 0.15mmol, 1.5eq.), dibenzoyl peroxide (2.4mg, 0.01mmol, 0.1eq.) and tert-butyl nitrite (20.6mg, 0.2mmol, 2.0eq.), replace the nitrogen three times, inject 0.6mL acetonitrile into the reaction tube under the nitrogen flow environment, screw the cap of the reaction tube tightly, stir vigorously at 80°C for 8 hours, stop the reaction, wait until the reaction solution drops to room temperature. The reaction solution was first filtered with diatomaceous earth to remove the reaction residue, the filter cake was washed three times with ethyl acetate, the filtrates were combined, spin-dried to obtain the target white solid 20.6 mg by column chromatography, and the yield was 75%.
[0034] The mechanism formula of the target product is:
[0035] The target product was analyzed by melting point, infrared and NMR mass...
Embodiment 2
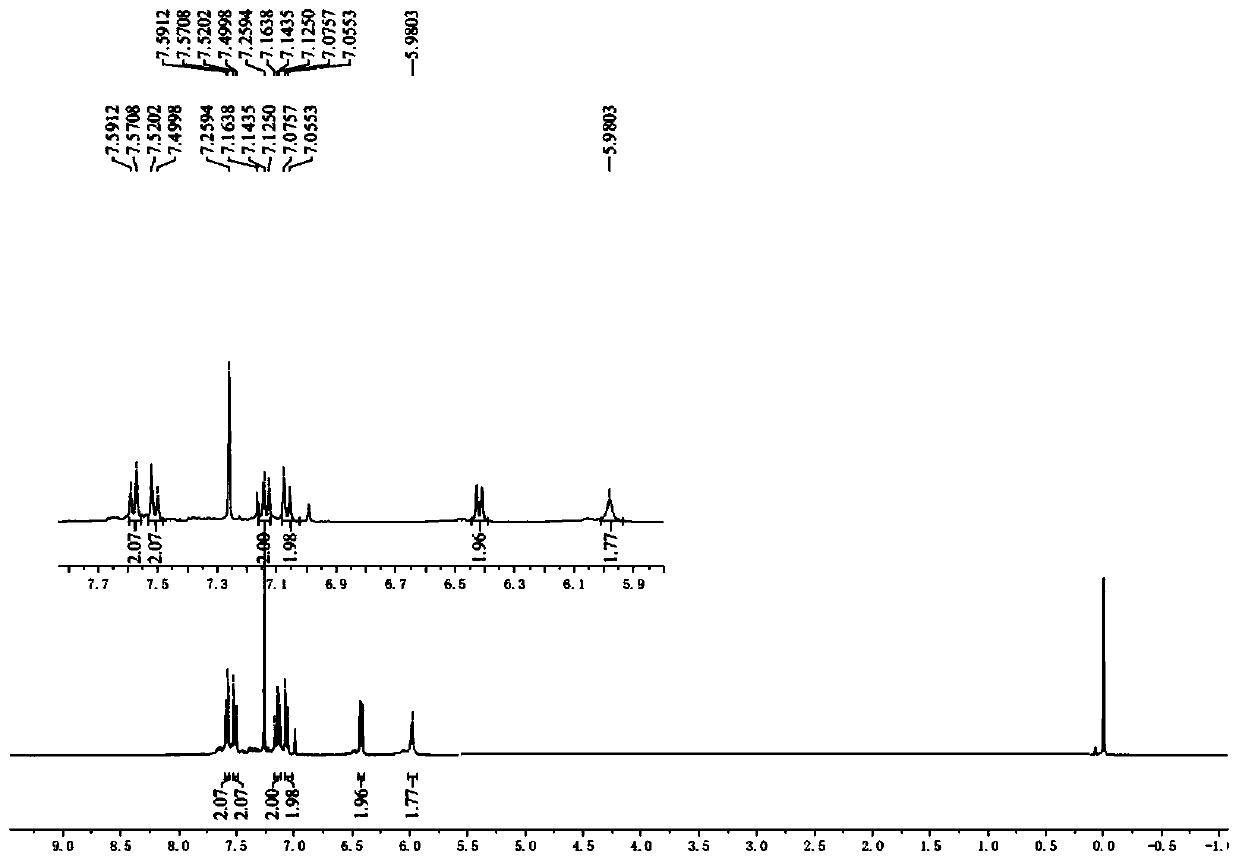
[0043] Add 4-bromoaniline (34.2mg, 0.2mmol, 2.0eq.), Bpin-Bdan (29.4mg, 0.1mmol, 1.0eq.), tetrabutylammonium iodide (3.7mg, 0.01 mmol, 0.1eq.), sodium acetate (12.3mg, 0.15mmol, 1.5eq.), dibenzoyl peroxide (2.4mg, 0.01mmol, 0.1eq.) and tert-butyl nitrite (20.6mg, 0.2mmol , 2.0eq.), replace the nitrogen three times, inject 0.6mL acetonitrile into the reaction tube under the nitrogen flow environment, tighten the cap of the reaction tube, stir vigorously at 80°C for 8 hours, stop the reaction, and wait for the reaction solution to drop to room temperature. The reaction solution was firstly filtered with diatomaceous earth to remove the reaction residue, the filter cake was washed three times with ethyl acetate, the filtrates were combined, and the solvent was spin-dried to obtain 20.0 mg of a white solid with a yield of 62% by column chromatography.
[0044] The mechanism formula of the target product is:
[0045] The target product was analyzed by infrared and NMR mass spect...
Embodiment 3
[0051] Add 2-methylaniline (21.4mg, 0.2mmol, 2.0eq.), Bpin-Bdan (29.4mg, 0.1mmol, 1.0eq.), tetrabutylammonium iodide (3.7mg, 0.01mmol, 0.1eq.), sodium acetate (12.3mg, 0.15mmol, 1.5eq.), dibenzoyl peroxide (2.4mg, 0.01mmol, 0.1eq.) and tert-butyl nitrite (20.6mg, 0.2 mmol, 2.0eq.), replace the nitrogen three times, inject 0.6mL acetonitrile into the reaction tube under the nitrogen flow environment, tighten the cap of the reaction tube, stir vigorously at 80°C for 8 hours, stop the reaction, and wait for the reaction solution to drop to room temperature . The reaction solution was first filtered with diatomaceous earth to remove the reaction residue, the filter cake was washed three times with ethyl acetate, the filtrates were combined, and the solvent was spin-dried to obtain 11.6 mg of a white solid with a yield of 45% by column chromatography.
[0052] The mechanism formula of the target product is:
[0053] The target product was analyzed by melting point, infrared and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com