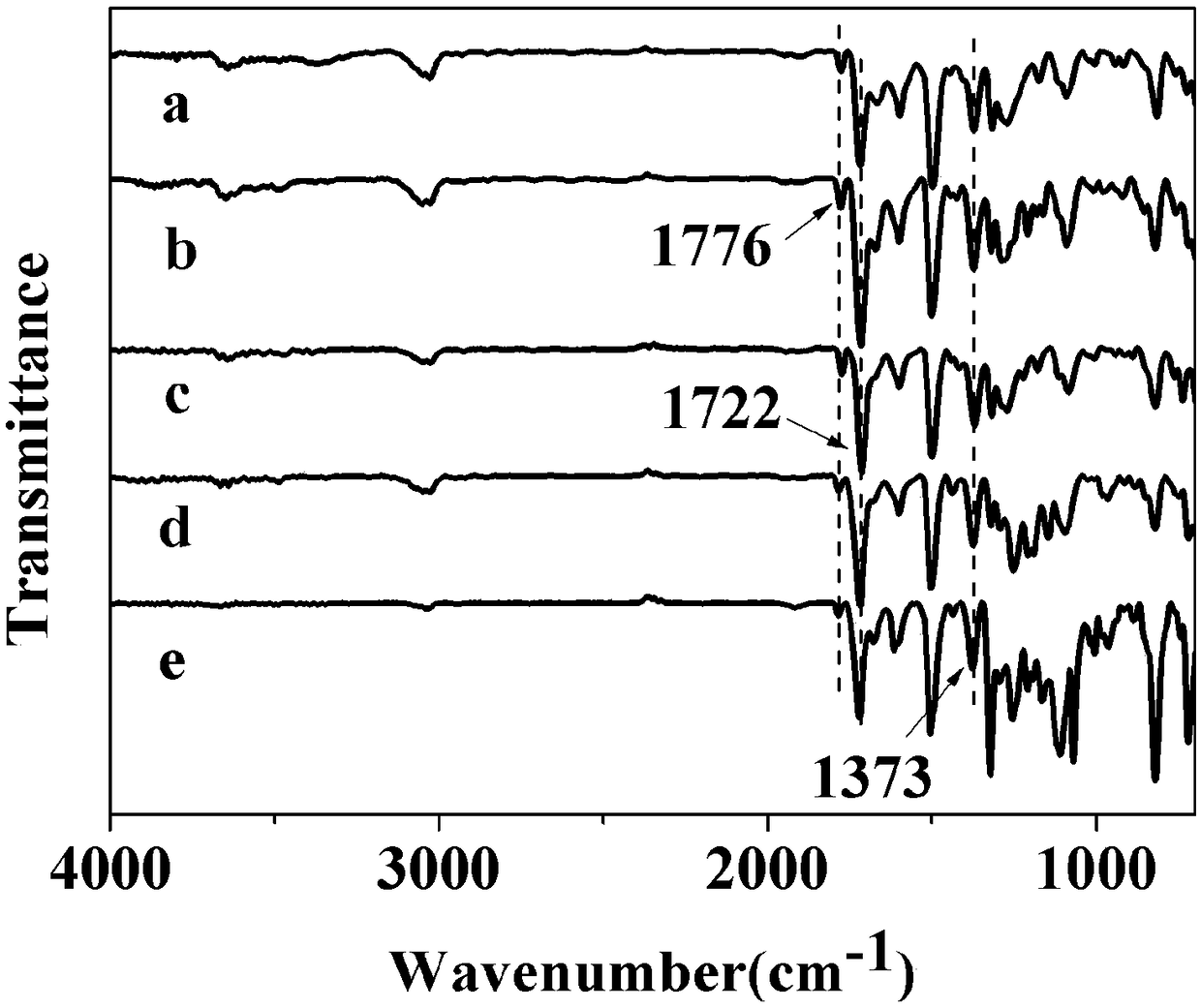
Aromatic hyperbranched polyimide containing anthracene structure and preparation method and application of aromatic hyperbranched polyimide
A technology of polyimide and polyimide film, which is applied in the field of material science, can solve the problems of restricting wide application, large distance between chains, and decreased heat resistance, so as to achieve good application prospects, improve heat resistance, increase Effect of large free volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
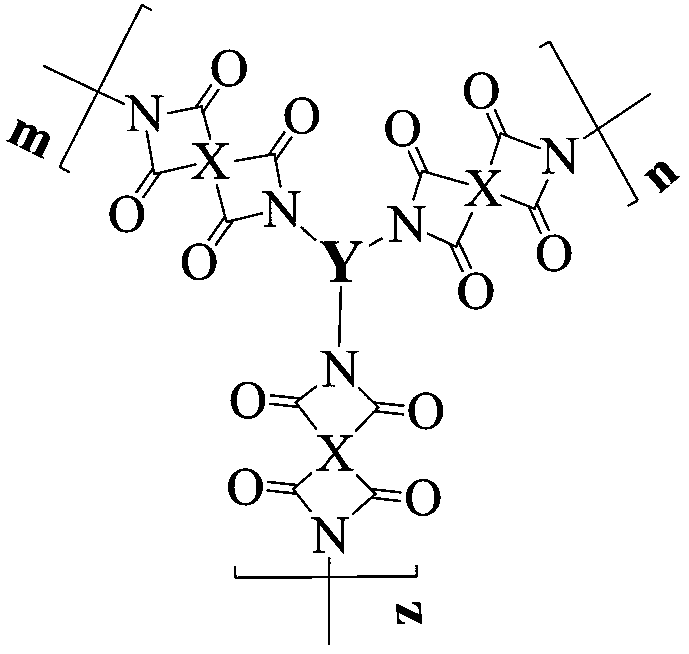
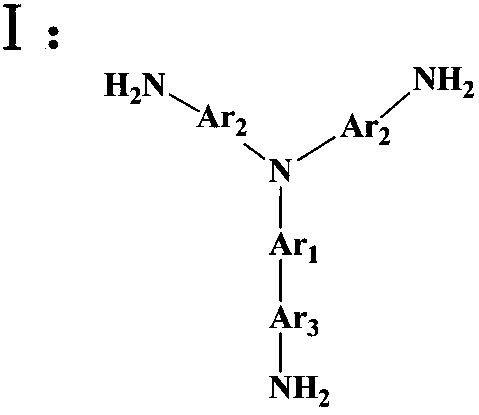
[0037] Add 0.4362g (2mmol) of pyromellitic dianhydride (PMDA) and 36ml of N,N-dimethylformamide into a three-necked flask, blow in argon, raise the temperature to 30°C, and add the triamine monomer N 1 -(4-aminophenyl)-N 1 -(9-(4-aminophenyl)anthracen-2-yl)benzene-1,4-diamine 0.4666g (1mmol) dissolved in 40ml of N,N-dimethylformamide with a constant pressure dropping funnel in 1 ~ 2h Add it dropwise into a three-necked flask, then continue to react for 13h, then add 6ml of acetic anhydride and 2ml of triethylamine, heat up to 45°C and continue to react for 12h, after the reaction is completed, cool to room temperature and discharge in methanol, filter, wash, repeat 2-3 Finally, place it in a vacuum drying oven at 80°C for 24 hours to obtain a reddish-brown hyperbranched polyimide polymer, whose structural formula is as follows:
[0038]
Embodiment 2
[0040] Add 0.903g (4.14mmol) of pyromellitic dianhydride (PMDA) and 2ml of N,N-dimethylacetamide into a three-necked flask, blow in argon, raise the temperature to 30°C, and add the triamine monomer N 1 -(4-aminophenyl)-N 1 -(10-(4-aminophenyl)anthracen-9-yl)benzene-1,4-diamine 0.9332g (2mmol) dissolved in 3ml N,N-dimethylacetamide with a constant pressure dropping funnel in 1 ~ 2h Add it dropwise into a three-necked flask, then continue to react for 16 hours, then add 12.4ml of acetic anhydride and 4.2ml of triethylamine, heat up to 45°C and continue to react for 13 hours, after the reaction is completed, cool to room temperature and discharge in ethanol, filter, wash, repeat 2 ~3 times, and finally placed in a vacuum drying oven at 80°C for 24 hours to obtain a light yellow hyperbranched polyimide polymer, whose structural formula is as follows:
[0041]
Embodiment 3
[0043]Add 0.4413g (1.5mmol) of 3,3',4,4'--biphenyltetracarboxylic dianhydride (BPDA) and 10ml of N-methylpyrrolidone into a three-necked flask, pass in argon, raise the temperature to 30°C, and Triamine Monomer N 2 -(9-(4-aminophenyl)anthracen-2-yl)-N 2 -(5-aminothiophen-2-yl)thiophene-2,5-diamine 0.4786g (1mmol) dissolved in 8ml of N-methylpyrrolidone was added dropwise into the three-necked flask with a constant pressure dropping funnel in 1~2h, and then continued to react for 24h , then add 12ml of acetic anhydride and 3ml of triethylamine, raise the temperature to 45°C and continue the reaction for 24h, after the reaction is completed, cool to room temperature and discharge the material in methanol, filter, wash, repeat 2-3 times, and finally place in a vacuum oven at 80°C Dry 24h in middle, obtain brown hyperbranched polyimide polymer, its structural formula is as follows:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com