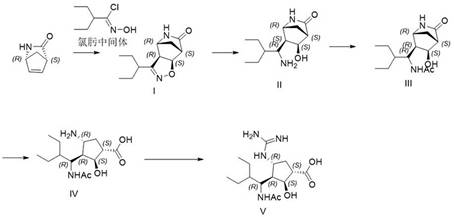
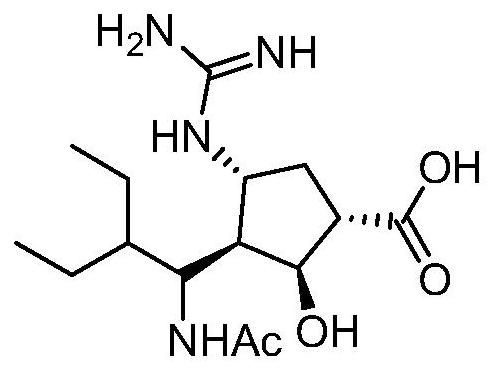
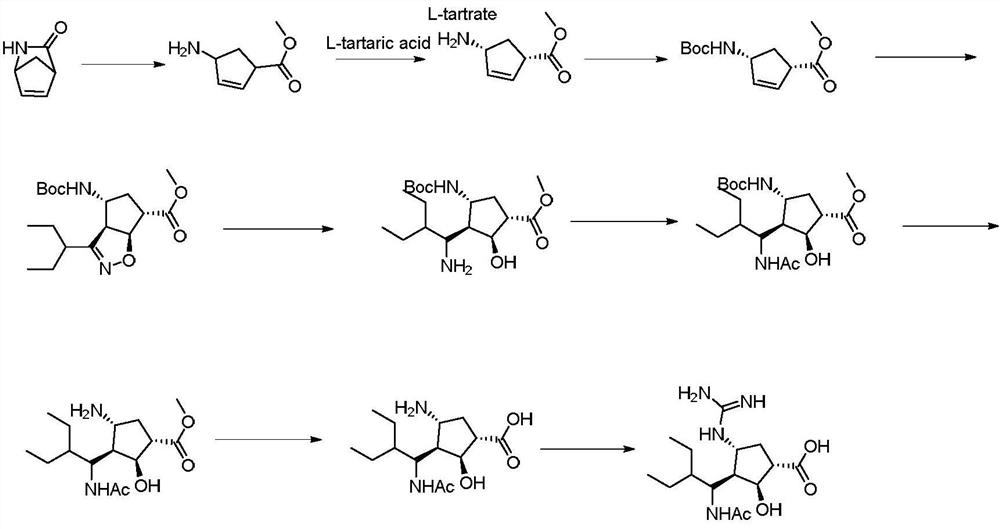
A kind of synthetic method of 3+2 ring closure
A synthetic method and technology of synthetic route, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low selectivity, increased cost, low yield, etc., to shorten the reaction steps and reduce the three wastes. The effect of reducing emissions and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Add (-)-wens lactone (1mmol) to a 250ml three-necked flask, add triethylamine (3mmmol), after stirring well, add the toluene solution (2mmol) of the newly prepared chloroxime intermediate, and stir the reaction solution at room temperature , add water after the reaction is over, separate layers, take the toluene phase, wash the toluene phase twice with water, and let it stand for later use.
[0024]
[0025] Add the toluene solution containing compound I in the previous step into a 250ml three-necked flask, add nickel chloride hexahydrate (0.5mmol), dissolve with methanol, and cool to 0-5°C. Take another beaker, add sodium hydroxide (0.02mmol) and sodium borohydride (1.5mmol), dissolve in methanol and slowly drop into the three-necked flask, after the drop is complete, stir and react at room temperature for 12 hours. Samples were taken and the results analyzed by HPLC. After treatment, add ammonia water to quench the reaction, heat the layers at 40-50°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com