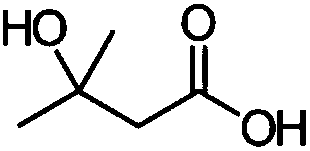
Method for preparing beta-hydroxy-beta-methylbutyric acid by enzymic method
A methylbutyric acid and amino acid technology is applied in the field of saccharopolyspora cytochrome P450 oxidase mutants to prepare beta-hydroxy-beta-methylbutyric acid, which can solve the difficulty of separation and purification and the complex components of reaction products and other problems, to achieve the effect of improving catalytic activity, easy post-treatment, and mild enzyme catalytic reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 P450 Oxidase Genetic Engineering Bacteria Construction
[0050] 1.1 Synthesize the sequence SEQ ID NO: 2 through the whole gene sequence, design the restriction site NdeI and BamHI at both ends, clone it into the corresponding site on the pSH plasmid, obtain the recombinant plasmid pSH-eryK, and then transform it into E. coli for expression by electroporation In the host BL21 (DE3) competent cells, coat the LB medium plate containing kanamycin, cultivate overnight at 37°C, pick a single colony, inoculate it into a test tube containing LB medium, cultivate overnight, collect the bacteria by centrifugation, and extract The plasmid was extracted, the gene sequence was confirmed to be correct, and the recombinant genetically engineered strain BL21(DE3) / pSH-eryK expressing wild-type eryK was obtained.
[0051] The amino acid sequence was determined as SEQ ID NO:1.
[0052] 1.2 Select a single clone from a plate containing eryK engineering strains, inoculate into 5...
Embodiment 2
[0053] Example 2 Error-prone PCR method constructs eryK random mutation point library
[0054]Using SEQ ID NO:2 as a template, an error-prone PCR technique was used to construct a random mutant library.
[0055] Forward primer eryK-Nde-F: 5'-CATATGACCACCATTGATGAAGTGCCGGG-3',
[0056] Reverse primer eryK-Bam-R: 5'-GGATCC TCAAGCACTCTGGCGCGGGCTACTA-3'.
[0057] 50μL error-prone PCR reaction system includes: 50ng plasmid template pSH-eryK, 30pmol a pair of primers eryK-Nde-F and eryK-Bam-R, 1X Taq buffer, 0.2mM dGTP, 0.2mM dATP, 1mM dCTP, 1mM dTTP, 7mM MgCl 2 , (0mM, 0.05mM, 0.1mM, 0.15mM, 0.2mM) MnCl 2 , 2.5 units of Taq enzyme (fermentas). The PCR reaction conditions were: 95°C for 5min; 94°C for 30s, 55°C for 30s, 72°C for 2min / kbp; 30 cycles; 72°C for 10min. The 2.0kb random mutation fragment was recovered from the gel as a large primer, and MegaPrimer PCR was performed with KOD-plus DNA polymerase: 94°C for 5min; 98°C for 10s, 60°C for 30s, 68°C for 2min / kbp, 25 cycles; ...
Embodiment 3
[0058] Example 3 High-throughput screening of eryK mutant library
[0059] 3.1 Select the transformant from the eryK mutant library and inoculate it into a 96-well deep-well culture plate containing 700 μL LB medium, which contains 100 μg / mL kanamycin, culture at 37°C for 6 hours, and then add a final concentration of 0.1 mM IPTG Afterwards, the temperature was lowered to 25°C and cultured overnight. Centrifuge at 5000rpm for 10min, discard the supernatant, freeze at -70°C for 1h, and thaw at room temperature for 30min. Add 200 μL of 0.1M potassium phosphate buffer (pH7.4) to resuspend the bacteria for the determination of eryK enzyme activity.
[0060] 3.2 Reagent preparation:
[0061] Substrate reaction solution: add 10 g / L of β-methylbutyric acid, adjust the pH with NaOH, and dissolve it.
[0062] Termination reaction solution: 0.5ml of 1M NaOH solution.
[0063] 3.3 Reaction process:
[0064] Add 80 μL of the substrate reaction solution to the 80 μL of the cell soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com