Method for preparing 4,5-diaryl-2H-1,2,3-triazole compound
A -2H-1, compound technology, applied in the direction of organic chemistry, can solve the problems of difficult separation and purification of the expected product, affecting the purity of the expected product, limiting the application value, etc., to achieve the effect of less impurities, avoiding self-coupling, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
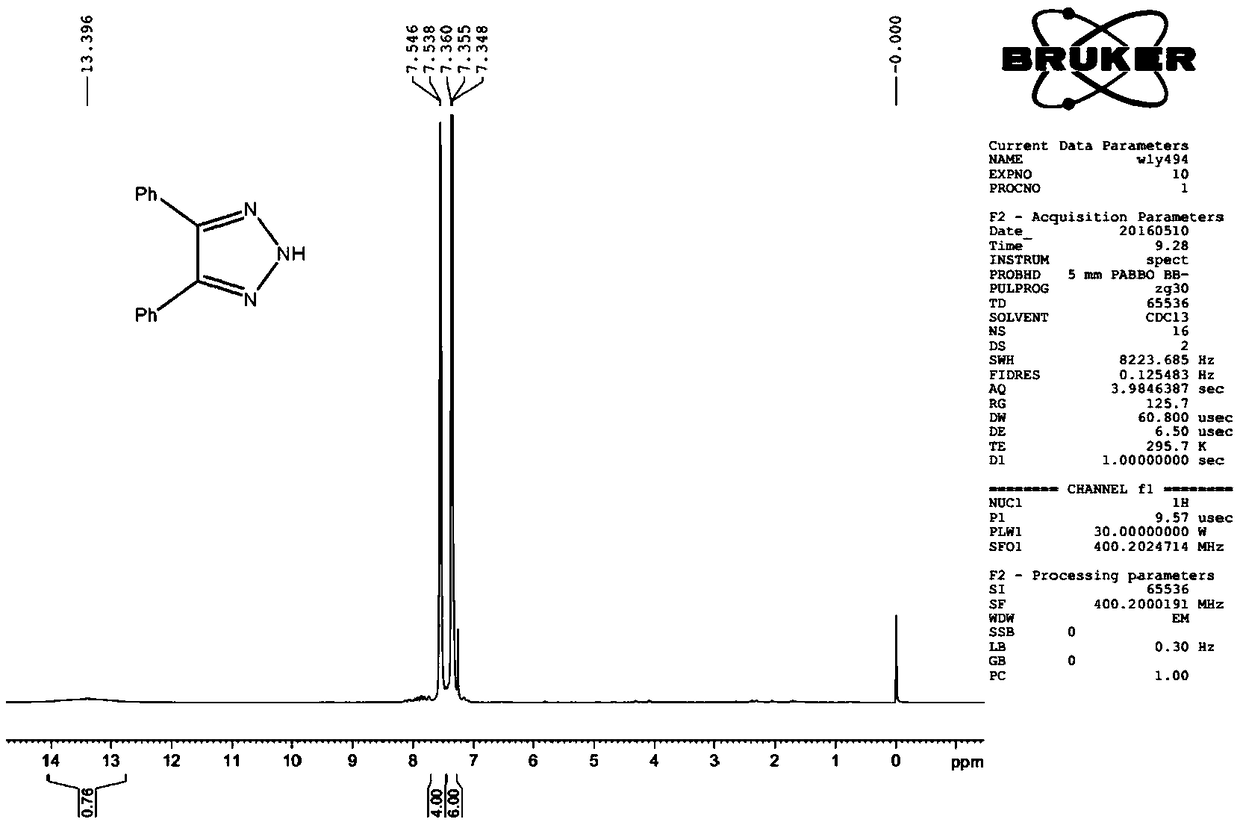
[0028] Example 1 Preparation of 4,5-diphenyl-2H-1,2,3-triazole
[0029]
[0030] Scheme 1: Dissolve 1.0mmol of benzaldehyde p-toluenesulfonylhydrazone and 1.2mmol of benzonitrile in 4.0mL of DMF, add 2.5mmol of potassium tert-butoxide under stirring, heat to 60°C, and react for 4 hours. TLC detects that the raw materials are almost completely disappeared, the reaction solution was cooled to room temperature, and after adding saturated ammonium chloride solution to quench the reaction, extracted with ethyl acetate, the organic phase was washed with water and saturated sodium chloride for 2-3 times, dried by adding anhydrous sodium sulfate, and filtered. After concentration, silica gel column chromatography (200-300 mesh silica gel), eluting with 20:1-2:1 (v:v) petroleum ether / ethyl acetate as eluent, gave 4,5-diphenyl -2H-1,2,3-triazole compound, 198 mg, white solid, yield 89.5%. 1 H NMR (CDCl 3 ,400MHz),δ:13.40(br,1H),7.54(d,J=3.2Hz,4H),7.37(m,6H). 13 C NMR (CDCl 3 , 10...
Embodiment 2
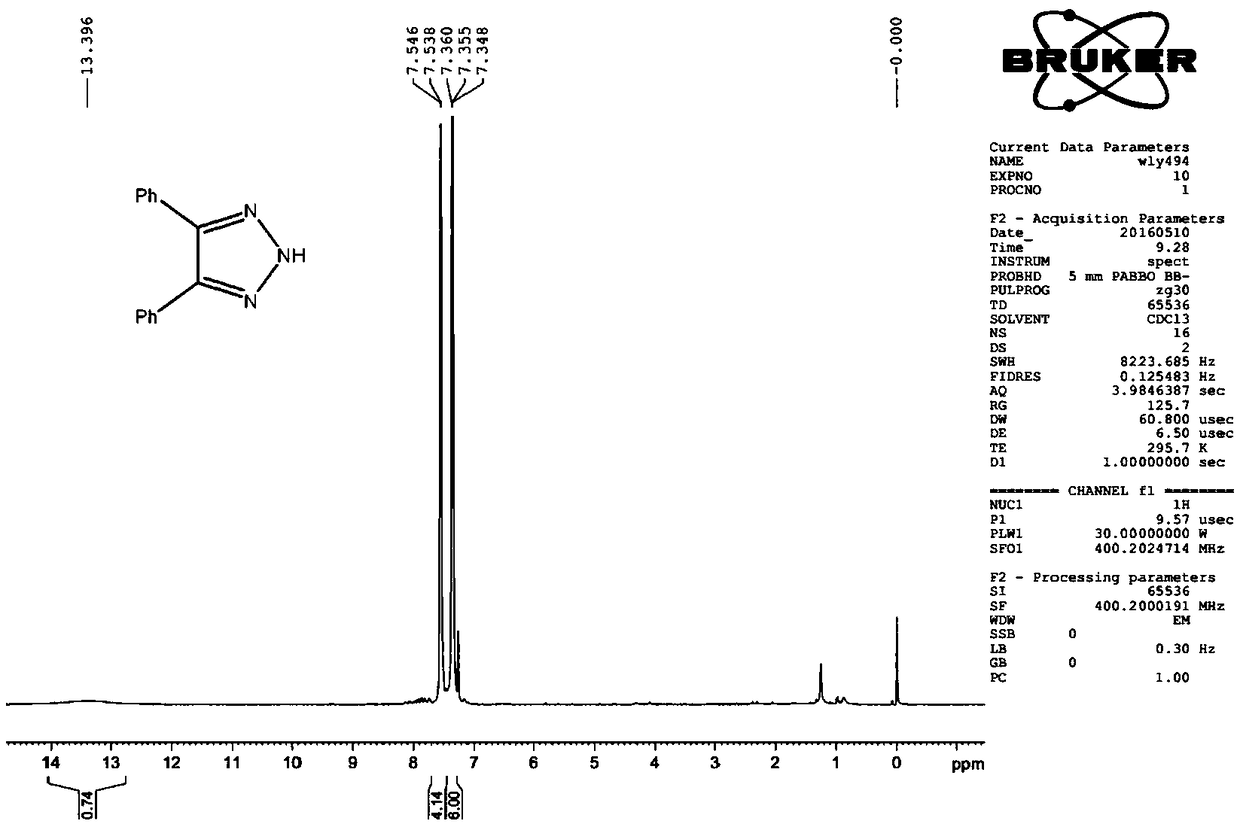
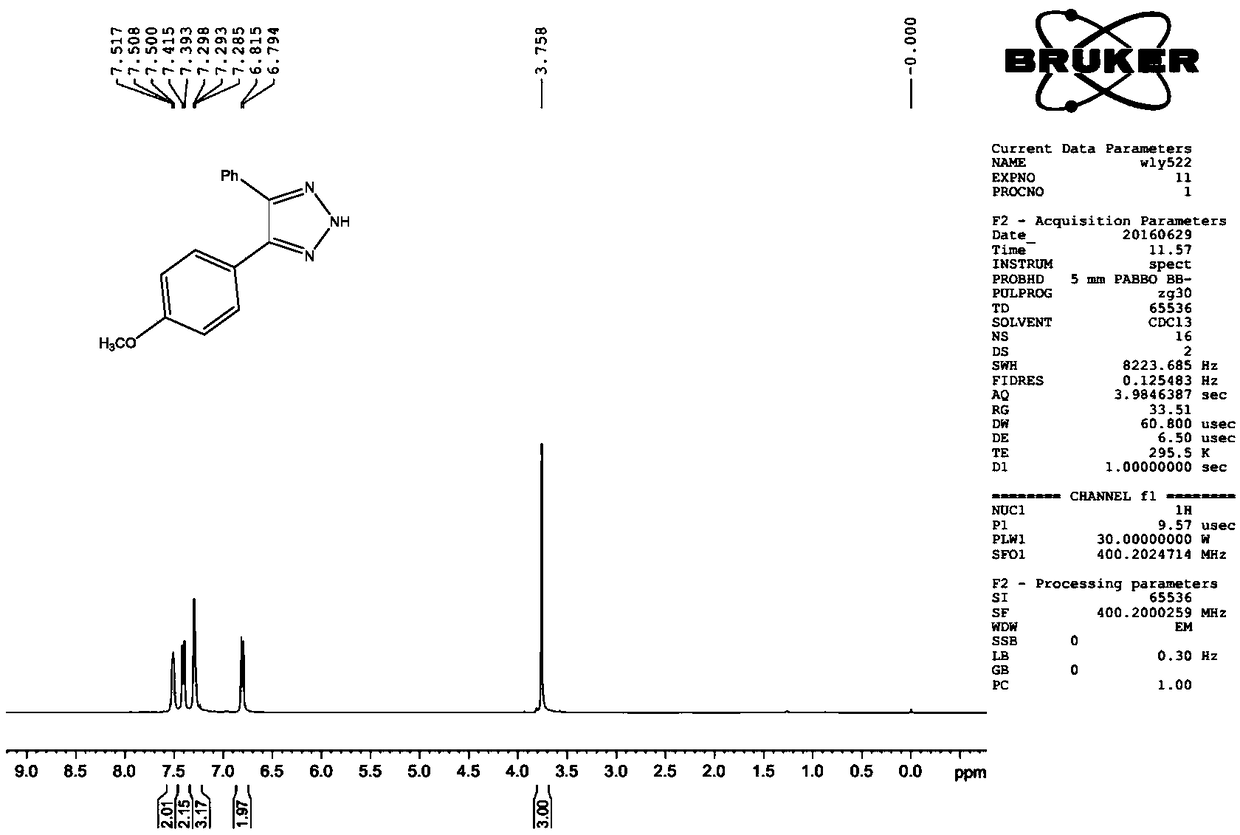
[0034] Example 2 Preparation of 4-(4-methoxyphenyl)-5-phenyl-2H-1,2,3-triazole
[0035]
[0036] Dissolve 1.0mmol of 4-methoxybenzaldehyde p-toluenesulfonylhydrazone and 1.0mmol of benzonitrile in 4.0mL of toluene, add 3.0mmol of NaHMDS under stirring, heat to 80°C, and react for 3 hours. TLC detects that the raw materials almost completely disappear , the reaction solution was cooled to room temperature, quenched the reaction by adding saturated ammonium chloride solution, extracted with ethyl acetate, washed the organic phase with water and saturated sodium chloride for 2-3 times, added anhydrous sodium sulfate to dry, filtered, and concentrated Afterwards, through silica gel column chromatography (200-300 mesh silica gel), use 20:1-2:1 (v:v) petroleum ether / ethyl acetate as eluent to obtain 4-(4-methoxybenzene Base)-5-phenyl-2H-1,2,3-triazole, colorless transparent glassy liquid, 221 mg, yield 87.9%. 1 H NMR (CDCl 3 ,400MHz),δ:7.50(t,J=3.2Hz,2H),7.40(d,J=8.8Hz,2H),7,29...
Embodiment 3
[0037] Example 3 Preparation of 4-phenyl-5-(3-trifluoromethyl)phenyl-2H-1,2,3-triazole
[0038]
[0039] Dissolve 1.0mmol of 3-trifluoromethylbenzaldehyde p-toluenesulfonylhydrazone and 1.2mmol of benzonitrile in 5.0mL of xylene, add 2.5mmol of NaHMDS under stirring, heat to 60°C, and react for 3 hours. TLC detects that the raw materials are almost completely disappeared, the reaction solution was cooled to room temperature, quenched by adding saturated ammonium chloride solution, extracted with ethyl acetate, the organic phase was washed with water and saturated sodium chloride for 2-3 times, dried by adding anhydrous sodium sulfate, and filtered , after concentration, through silica gel column chromatography (200-300 mesh silica gel), use 20:1-2:1 (v:v) petroleum ether / ethyl acetate as eluent to obtain 4-phenyl-5 -(3-trifluoromethyl)phenyl-2H-1,2,3-triazole, colorless transparent glassy liquid, 258 mg, yield 89.2%. 1 H NMR (CDCl 3 ,400MHz), δ:7.90(s,1H),7.73(d,J=7.6Hz,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com