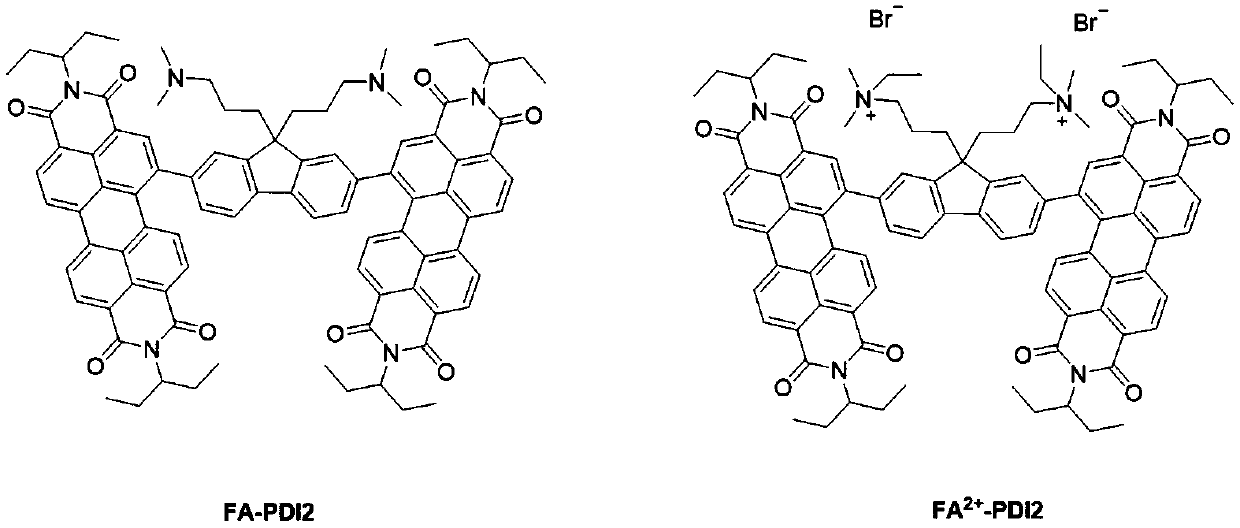
Ionic perylene diimide electron transport material and synthesis method and use thereof
An electron transport material, perylene diimide technology, applied in circuits, electrical components, electrical solid devices, etc., to achieve the effects of easy deployment, improved energy conversion efficiency, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: FA 2+ - Synthesis of PDI2:
[0038]
[0039] (1) 2,7-dibromo-9,9-bis[3'-(N,N-dimethylamino)propyl]-fluorene (1.5g, 3.0mmol), pinacol borate (1.9 g, 7.5mmol), [1,1-bis(diphenylphosphino)ferrocene]palladium dichloride (0.5g, 0.6mmol), potassium acetate (3.5g, 35.9mmol) were added to the reaction flask and dissolved in Tetrahydrofuran (100 mL) solution was stirred evenly under nitrogen protection, heated to 80° C., and reacted for 24 h. After the reaction was completed, it was cooled to room temperature, 100 mL of water was added to the reaction solution, extracted three times with 150 mL of ethyl acetate, the organic layer was collected, and the solvent was removed under reduced pressure. The residue was subjected to silica gel column chromatography, methanol / dichloromethane (1:20) was used as the eluent, separated and purified, and after vacuum drying, the target product 2,7-bis(4,4,5,5-tetra Methyl-1,3,2-dioxaborolan-2-yl)-9,9-bis[3'-(N,N-dimethylamino)...
Embodiment 2
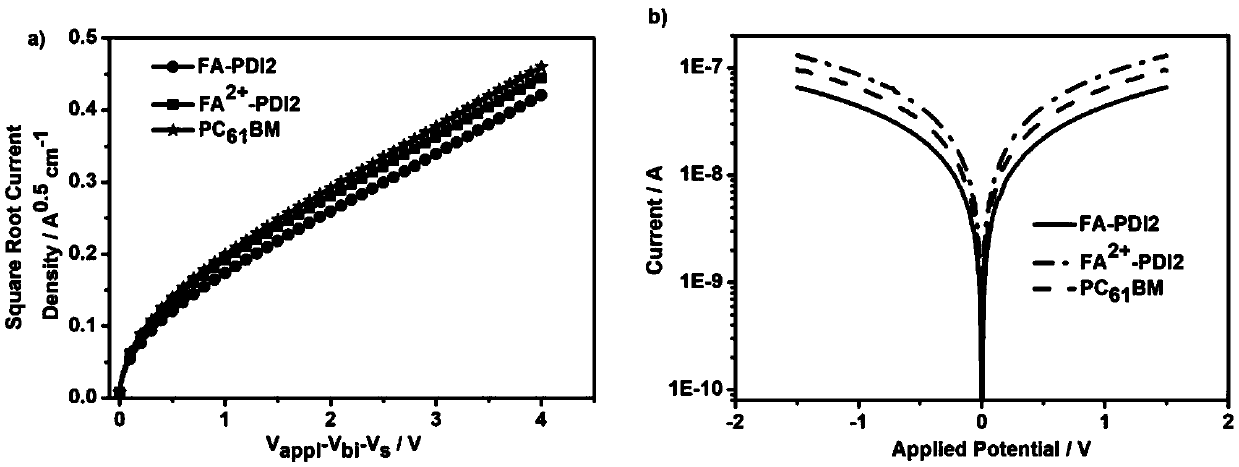
[0042] Embodiment 2: the synthetic compound FA of embodiment 1 2+ - Electron mobility and conductivity test of PDI2
[0043] Compound FA synthesized in embodiment 1 2+ -The electron mobility of PDI2 is tested by the space current limitation method (SCLC). The battery device structure used in the test is: F-doped tin oxide conductive glass / titanium dioxide / electron transport material / titanium dioxide / silver. For pure electronic devices , the space current limitation method test can be described as
[0044]
[0045] Among them, ε the dielectric constant of the electron transport material; ε 0 is the dielectric constant of vacuum; μ 0 is the zero-field mobility; J is the current density; L is the film thickness.
[0046] Compound FA synthesized in embodiment 1 2+ -The conductivity of PDI2 is tested using a two-probe conductivity test method, and the conductivity is determined by the following equation
[0047] s=L / Rwd
[0048] Among them, L is the channel length (10mm);...
Embodiment 3
[0049] Embodiment 3: With the synthetic compound FA of embodiment 1 2+ -Assembly of trans perovskite solar cells with PDI2 as electron transport material
[0050] FTO (fluorine doped tin dioxide) conductive glass was cut into 25mm x 15mm glass substrates. , and etch using zinc powder and hydrochloric acid chemistry. The etched glass substrate was cleaned in deionized water, acetone and ethanol for 15 minutes respectively. Using the spray pyrolysis method, the mixed solution of 0.02M nickel acetylacetonate in acetonitrile and ethanol (volume ratio of 95:5) was heated to 450°C and sprayed onto the glass substrate to form a very thin dense layer of nickel oxide, and then It was heated to 450°C and sintered for 30 minutes. The following operating steps (except the vacuum evaporation step) were all completed in a nitrogen-filled glove box. We will lead iodide (PbI 2 ), lead chloride (PbCl 2 ), methylammonium iodide (MAI) (molar ratio 0.93:0.17:1) was dissolved in N,N-dimethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com