A crystal form of a tubulin inhibitor (vda-1)
A crystal form, tert-butyl technology, applied in medical preparations containing active ingredients, drug combinations, organic chemistry, etc., can solve problems such as poor water solubility, unfavorable allergic reactions, and reduced curative effect, and achieve simple production and operation, chemical Good stability and convenient storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 [crude product preparation of VDA-1]
[0036] step 1)
[0037]
[0038] The synthesis of Intermediate A refers to the preparation method described in Example 2 Route A in Chinese Patent Application CN1684955.
[0039] step (2)
[0040]
[0041] 3-Fluorobenzaldehyde (0.10mol), formylmethylenetriphenylphosphine (33.5g, 0.11mmol) and toluene (200ml) were added to a 1L dry one-necked flask. The reaction was refluxed for 16 hours and then concentrated. The crude product was purified by column chromatography (eluent: volume ratio of petroleum ether / ethyl acetate: 100 / 1 to 80 / 20) to obtain 3-(3-fluorophenyl)acrolein (yield: 62%).
[0042] step (3)
[0043]
[0044]DMF (100ml), intermediate A (1.38g, 5mmol), 3-(3-fluorophenyl)acrolein (10mmol) and cesium carbonate (3.26g, 10mmol) were added to a 250ml dry one-necked flask. The reaction system was stirred at 25°C for 12 hours, cooled to room temperature, and poured into ice water. Extracted with ethyl...
Embodiment 2
[0045] Embodiment 2 [VDA-1 recrystallization process screening]
[0046] Process 1: Take 200g of crude VDA-1 and add it to the reaction flask, add 2400ml of ethyl acetate and tetrahydrofuran mixed solvent (V / V=1:5), heat up to reflux to 60°C while stirring. After dissolving, stir for 10 minutes, then lower the temperature to 5-15°C, stir and crystallize for 4 hours after solid precipitation, filter with suction, and rinse the filter cake with acetone. The filter cake was air-dried at 45°C and assisted with phosphorus pentoxide. 166 g of off-white solid was obtained, with a yield of 83.0%. Moisture was measured to be 0.3% with a Karl Fischer analyzer. (3Z,6Z)-3-[((E)-3-(5-tert-butyl)-1H-imidazolyl-4-yl)methylene]-6-((E)-3-(3-fluoro phenyl)-2-propenylidene)piperazine-2,5-dione (VDA-1).
[0047] Process 2: Take 200g of crude VDA-1 and add it to the reaction flask, add 1600ml of acetone and water mixed solvent (V / V=1:3), and heat up to reflux to 60°C while stirring. After dis...
Embodiment 3
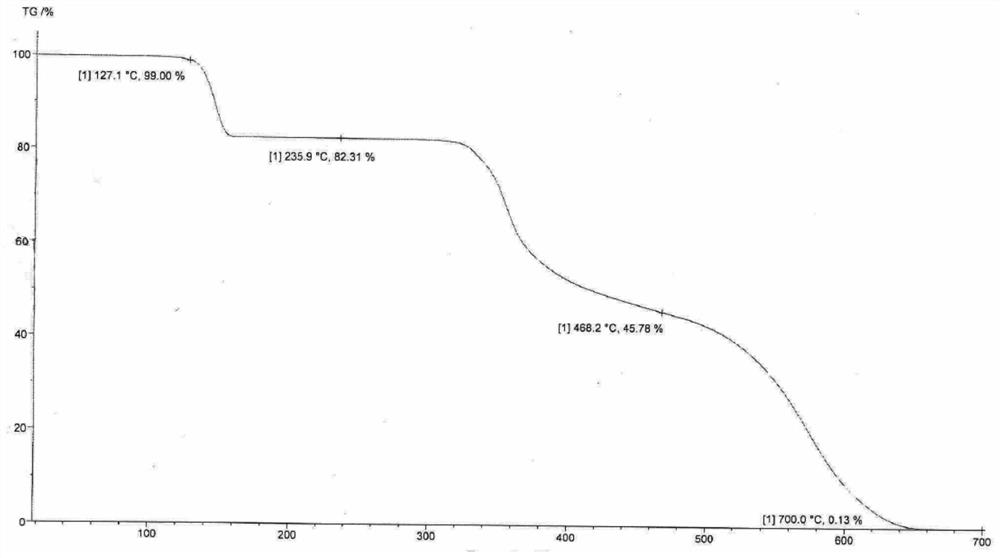
[0060] Embodiment 3 [detection of VDA-1 crystal form]
[0061] The test condition of embodiment sample:
[0062] 3.1 XRD:
[0063] Testing instrument: Empyrean X-ray diffractometer
[0064] Detection conditions: Cu target Kα ray, voltage 40kV, current 40mA, divergence slit 1 / 32°, anti-scatter slit 1 / 16°, anti-scatter slit 7.5mm, 2θ range: 3°-50°, step size 0.02 °, the residence time of each step is 40S.
[0065] Test basis: People's Republic of China (2015 Edition IV) 0451 X-ray powder diffraction method
[0066] Test result: if figure 1 shown.
[0067] 3.2DSC:
[0068] Testing instrument: DSC 214 differential scanning calorimeter from NETZSCH, Germany
[0069] Detection conditions: Atmosphere: N 2 , 40ml / min
[0070] Scanning program: from room temperature to 250°C at 10°C / min, record the temperature rise curve.
[0071] Test sample quality: sample 1: 2.48mg (using aluminum sample pan)
[0072] Testing basis: JY / T 014-1996 General Rules for Thermal Analysis Methods...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com