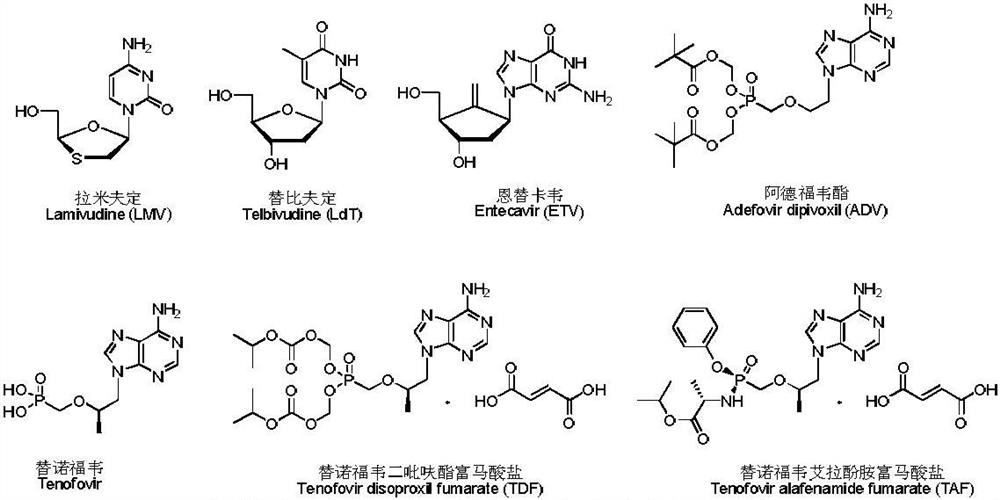
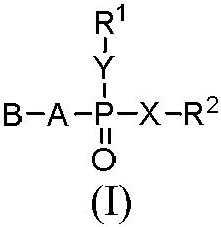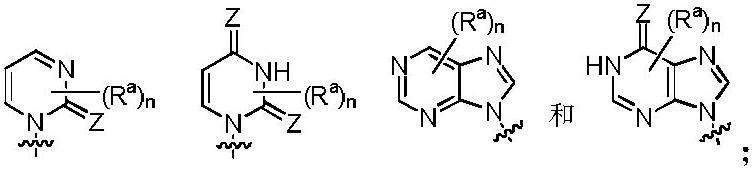Heterocyclic compound and its preparation method and use
A compound and cycloalkyl technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as unsatisfactory activity and improve drug metabolism Kinetic properties, good physical and chemical properties, and the effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0241] The present invention is further described below in conjunction with the examples, but the provision of these examples is not intended to limit the scope of the present invention.
[0242] Unless otherwise stated, commercially available anhydrous and HPLC grade solvents were used without further purification.
[0243] Recorded at ambient temperature with a Bruker instrument (400MHz) 1 H NMR spectrum using TMS as internal standard. Chemical shifts (δ) are given in ppm and coupling constants (J) are given in Hertz (Hz). 1 The splitting multiplicity of H NMR spectral peaks is abbreviated as follows: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), br (broad).
[0244] LC-MS adopts Agilent 1200 liquid chromatograph coupled with Agilent 6120 Quadrupole mass spectrometer, and detects at 214nm and 254nm. The preparative liquid chromatography uses SHIMADZU CBM-20A and Agilent 1260 preparative liquid chromatograph, C18OBD 19×150mm5μM preparative column, dete...
Embodiment 1
[0246] Example 1 (4R)-6-((4-(4-((((((R)-1-(6-amino-9H-purin-9-yl)prop-2-yl)oxy)methyl Base) ((2-methylbenzyl)oxy)phosphono)oxy)phenyl)piperidin-1-yl)methyl)-4-(2-chloro-4-fluorophenyl)-2- (Thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylic acid ethyl ester (Compound 1)
[0247]
[0248] Step 1: Synthesis of tert-butyl 4-(4-hydroxyphenyl)piperidine-1-carboxylate (compound 1-2)
[0249] At room temperature, tert-butyl 4-(4-(benzyloxy)phenyl)-3,6-dihydropyridine-1(2H)-carboxylate (compound 1-1) (3.0g, 8.2mmol) was added Add 10% Pd / C (300mg) to tetrahydrofuran (10mL) and methanol (20mL), after the compound is completely dissolved, add 10% Pd / C (300mg) under the protection of nitrogen, complete the addition, replace with hydrogen, react at room temperature for 18h, filter, and post-process the filtrate to obtain The title compound (2.0 g). ESI-MS(m / z): 222.1[M-55+H] + .
[0250] Step 2: 4-(4-(((((R)-1-(6-amino-9H-purin-9-yl)prop-2-yl)oxy)methyl)(hydroxy)phosphono) Synthesis ...
Embodiment 2
[0260] Example 2 (4R)-6-((4-(4-((((((R)-1-(6-amino-9H-purin-9-yl)prop-2-yl)oxy)methyl base)(((S)-1-ethoxy-1-oxopropan-2-yl)oxy)phosphono)oxy)phenyl)piperidin-1-yl)methyl)-4-( 2-Chloro-4-fluorophenyl)-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylic acid ethyl ester (Compound 2)
[0261]
[0262] Compound 1-5 (30 mg, 0.04 mmol), L-ethyl lactate (8.0 mg, 0.07 mmol) and PyBOP (38 mg, 0.07 mmol) were dissolved in N,N-dimethylformamide (3 mL) at room temperature , after the dissolution was complete, N,N-diisopropylethylamine (19 mg, 0.14 mmol) was added dropwise. After the addition was complete, the reaction was carried out at room temperature for about 4 h, and the title compound (5.0 mg) was obtained after purification.
[0263] Its structure is characterized as follows:
[0264] 1 H NMR (400MHz, DMSO-d 6 )δ9.77(s, 1H), 8.13(s, 1H), 8.05(s, 1H), 8.03(d, J=23.26Hz, 1H), 7.95(d, J=3.13Hz, 1H), 7.44- 7.40(m, 2H), 7.27-7.16(m, 5H), 7.05-7.03(m, 2H), 6.06(s, 1H), 5.00-4.92(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com