Hydrogen storage material hydrolysis and hydrogen releasing system taking monodispersed or supported phosphorus-containing metal compound as catalyst
A hydrogen storage material, monodisperse technology, applied in the field of hydrogen storage material water interpretation hydrogen system, can solve the problems that restrict the commercialization of catalysts, the instability of nanoparticles to oxygen, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
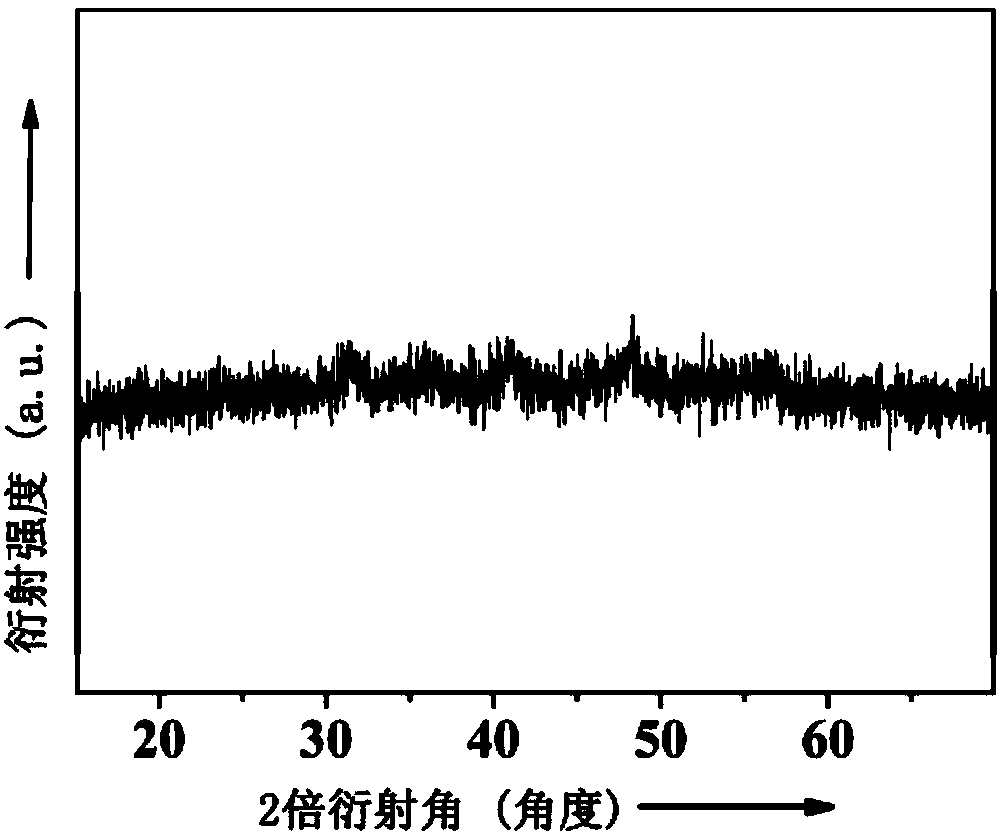
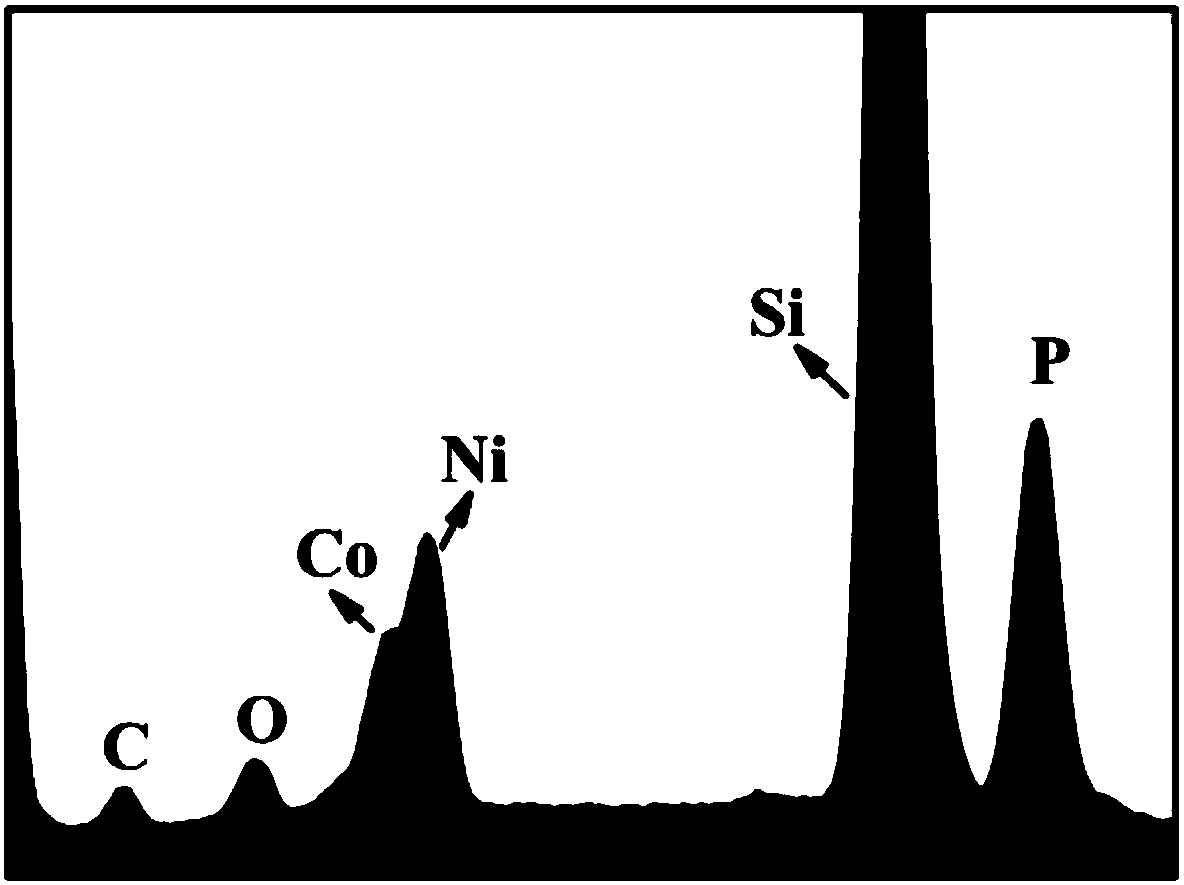
[0099] A kind of cobalt nickel phosphide (Ni 0.7 co 1.3 P) the preparation method of catalyst, comprises the steps:
[0100] 1) Mix 250mg sodium citrate, 2.0g sodium hydroxide and 80mL distilled water evenly to obtain a mixed solution;
[0101] 2) Dissolve 350 mg of nickel nitrate hexahydrate and 650 mg of cobalt nitrate hexahydrate in 20 mL of distilled water to obtain a metal salt solution; slowly add the metal salt solution into the mixture, stir at room temperature for 1 h after the addition is complete, then centrifuge to collect the precipitate and use Washing with distilled water, and fully drying at 373K to remove water to obtain the precursor;
[0102] 3) 200 mg of the precursor and 1000 mg of phosphate were fully mixed and ground, and after grinding, the powder was placed in a tube furnace, and the tube furnace was heated from room temperature to 573K at a heating rate of 5K / min under an argon flow, and the Carry out calcination at this temperature for 2 hours, th...
Embodiment 2
[0105] A kind of cobalt nickel phosphide (Ni 0.7 co 1.3 P) the preparation method of catalyst, comprises the steps:
[0106] 1) Mix 350mg nickel nitrate hexahydrate, 650mg cobalt nitrate hexahydrate and 20mL distilled water evenly to obtain a mixed solution;
[0107] 2) Add 1g of red phosphorus to the mixed solution, then carry out hydrothermal reaction at 473K for 20h; cool to room temperature after the reaction, collect the precipitate by centrifugation and wash the precipitate with distilled water to obtain nickel cobalt phosphide (Ni 0.7 co 1.3 P).
Embodiment 3
[0109] A kind of cobalt nickel phosphide (Ni 0.7 co 1.3 P) the preparation method of catalyst, comprises the steps:
[0110] 1) Mix 350mg nickel nitrate hexahydrate, 650mg cobalt nitrate hexahydrate and 20mL distilled water evenly to obtain a mixed solution;
[0111] 2) Add 400mg of white phosphorus to the mixed solution, then carry out hydrothermal reaction at 423K for 5h; cool to room temperature after the reaction, collect the precipitate by centrifugation and wash the precipitate with distilled water to obtain nickel cobalt phosphide (Ni 0.7 co 1.3 P).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com