Hyperbranched scale-inhibiting polymer as well as preparation method and application thereof
A technology of hyperbranched polyether and polymer, applied in chemical instruments and methods, descaling and water softening, water/sludge/sewage treatment, etc. Scale inhibition performance decline and other problems, to achieve the effect of eliminating easy hydrolysis, long duration, mild experimental conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation method of hyperbranched antiscaling polymer comprises the steps:
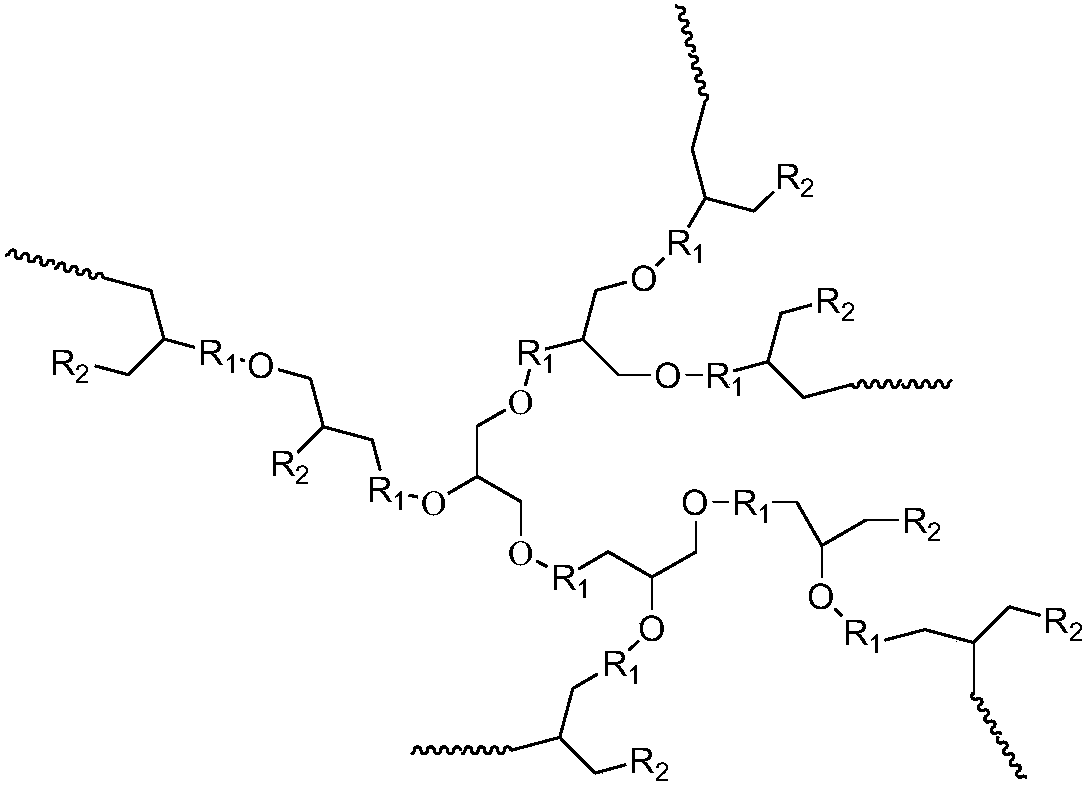
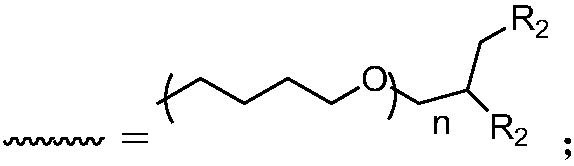
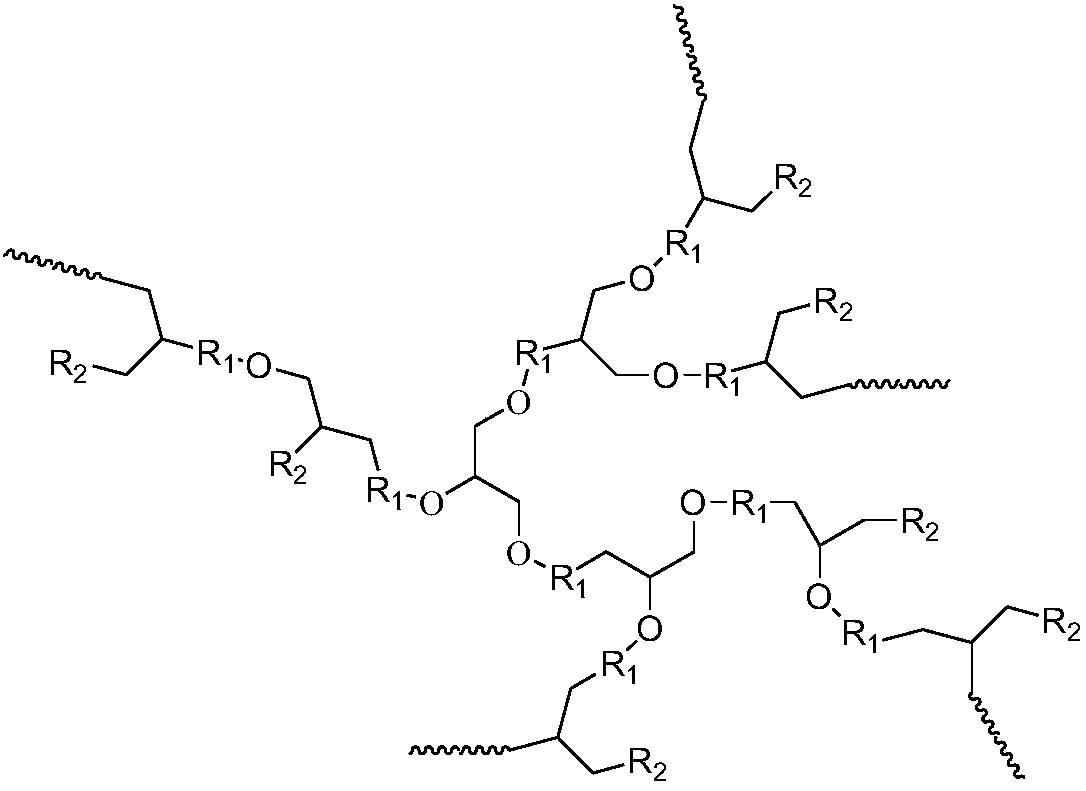
[0051] 1) Synthesis of hyperbranched polyether: Under ice-water bath conditions, 100 parts by volume of tetrahydrofuran and 30 parts by volume of glycidol are dissolved in the organic solvent toluene, and after stirring and mixing evenly, adding trifluoromethanesulfonate with a volume part of 0.1 acid, carry out cationic ring-opening copolymerization under normal pressure for 5 hours, add an aqueous solution of sodium hydroxide 10 times the volume of the cationic initiator to terminate the reaction, and obtain a hyperbranched polyether.
[0052] 2) Synthesis of hyperbranched antiscalant polymer: add 1 molar mass part of hyperbranched polyether, 100 molar mass parts of phosphoric anhydride and 0.001 molar mass part of catalyst 4-dimethylaminopyridine (DMAP), react at 60°C for 12h, During the reaction process, nitrogen gas was heated to the same temperature as the reaction system, and passe...
Embodiment 2
[0055] The preparation method of hyperbranched antiscaling polymer comprises the steps:
[0056] 1) Synthesis of hyperbranched polyether: at room temperature, 80 volume parts of tetrahydrofuran and 50 volume parts of glycidol were dissolved in acetone, and after stirring and mixing evenly, 0.5 volume parts of cationic initiator BF was added 3 , carry out the cationic ring-opening copolymerization reaction under normal pressure for 0.5h, add the terminator potassium hydroxide aqueous solution which is 500 times the volume of the cationic initiator to terminate the reaction, and obtain the hyperbranched polyether.
[0057] 2) Synthesis of hyperbranched scale-inhibiting polymer: add hyperbranched polyether with molar mass parts of 2.5, phosphoric acid with molar mass parts of 500, and 4-dimethylaminopyridine (DMAP) with molar mass parts of catalyst of 0.005, molar mass parts of 0.005 of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), reacted at 80°C for 6h, dur...
Embodiment 3
[0060] The preparation method of hyperbranched antiscaling polymer comprises the steps:
[0061] 1) Synthesis of hyperbranched polyether: at 0°C to 5°C, dissolve 100 volume parts of tetrahydrofuran and 50 volume parts of glycidol in dichloromethane, stir and mix evenly, and then add 0.25 volume parts of cationic initiator Carry out cationic ring-opening copolymerization of boron trifluoride ether under normal pressure for 5 hours, add a terminator water 500 times the volume of the cationic initiator to terminate the reaction, and obtain a hyperbranched polyether.
[0062] 2) Synthesis of hyperbranched scale-inhibiting polymer: add hyperbranched polyether with a molar mass of 1, 2-phospho-1,2,4-tricarboxylic butane (PBTCA) with a molar mass of 250, and molar mass parts 0.005 catalyst 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), react at 70°C for 10h, during the reaction, heat the nitrogen to the same temperature as the reaction system , into the reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average corrosion rate | aaaaa | aaaaa |
| Average corrosion rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com