Chimeric antigen receptor immune cell as well as preparation method and application thereof
A chimeric antigen receptor and immune cell technology, applied in the field of chimeric antigen receptor immune cells and its preparation, can solve the problems of T cell exhaustion, difficulty in obtaining therapeutic effect, limited number of TILs, etc., and avoid immune cell apoptosis , enhanced lethality, moderate affinity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1 plasmid construction
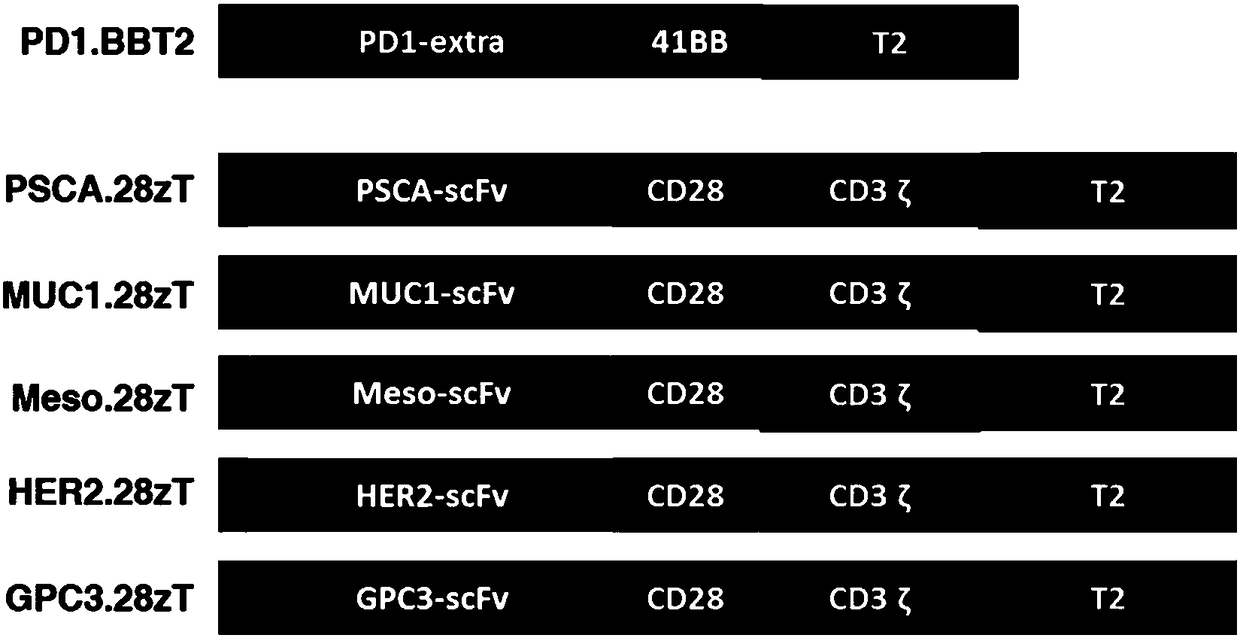
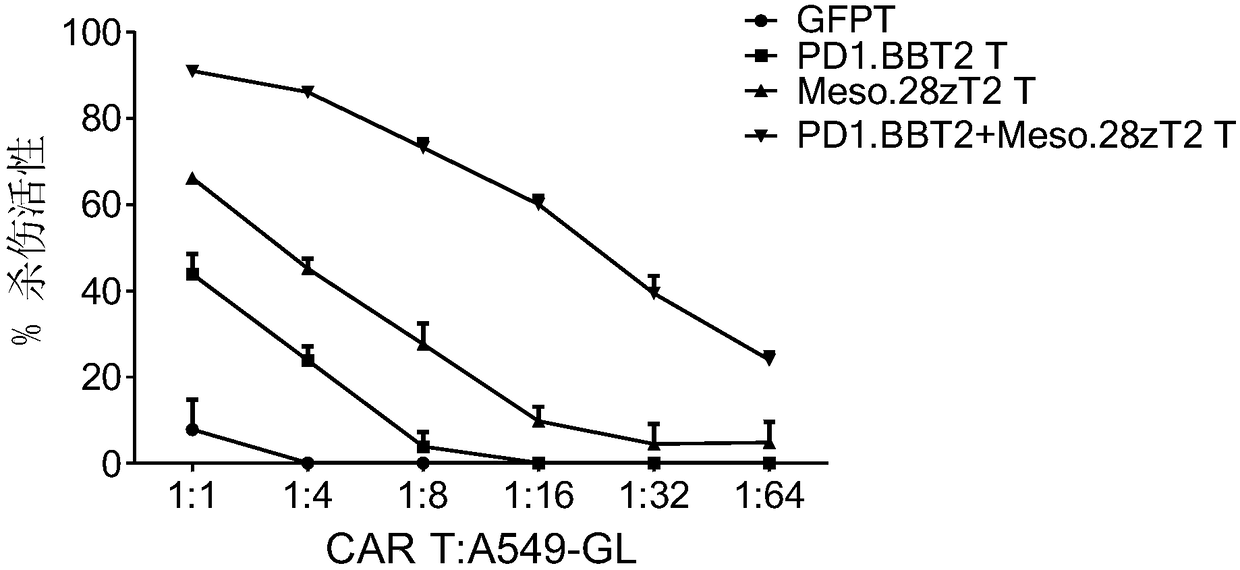
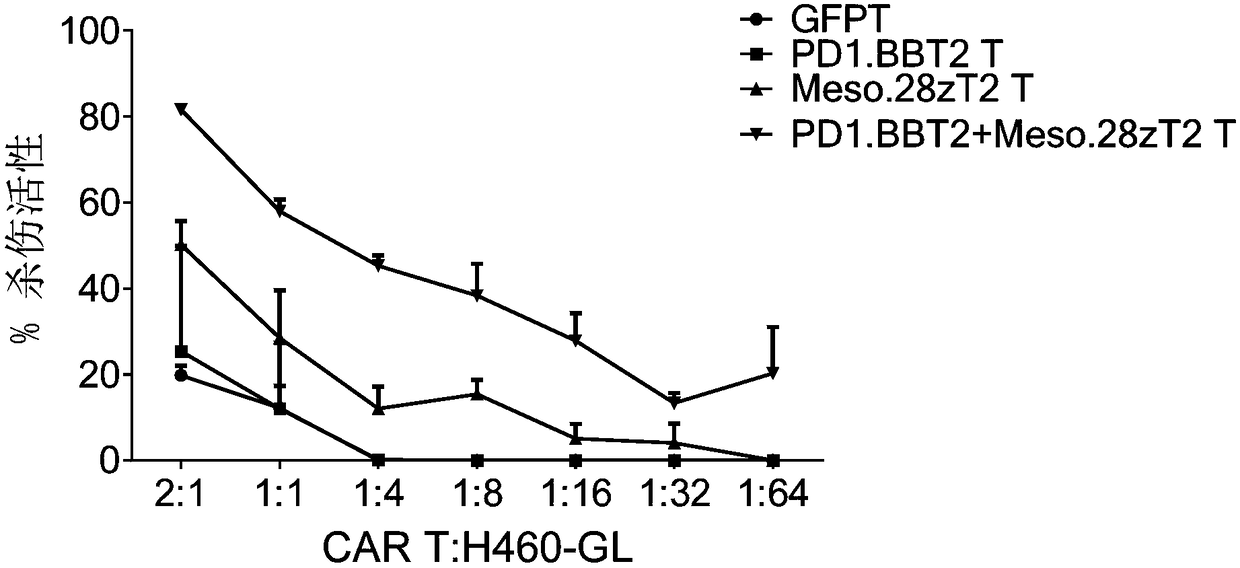
[0115] (1) if figure 1 Shown are anti-PD-L1 chimeric antigen receptor (PD1.BBT2), anti-Mesothelin chimeric antigen receptor (Meso.28zT2), anti-PSCA chimeric antigen receptor (PSCA.28zT2), anti-MUC1 chimeric antigen receptor (MUC1.28zT2), anti-HER2 chimeric antigen receptor (HER2.28zT2) and anti-GPC3 chimeric antigen receptor (GPC3.28zT2) molecular structure, synthetic nucleic acid sequence of the above chimeric antigen receptor, such as SEQ ID As shown in NO.1-15, the 3' end of all synthetic sequences contains a restriction endonuclease PmeI cutting site, and the 5' end contains a restriction endonuclease SpeI cutting site;
[0116] (2) Restriction endonucleases PmeI and SpeI were used to double-digest the nucleic acid sequences of the lentiviral expression vector pwpxld-eGFP and the synthetic chimeric antigen receptor;
[0117] (3) Agarose gel electrophoresis to recover target genes containing cohesive ends: PD1.BBT2, Meso.28zT2, P...
Embodiment 2
[0119] Example 2 lentiviral packaging
[0120] Use 293T cells for virus packaging, and when the cell confluence reaches 80-90%, perform lentivirus packaging:
[0121] (1) 2 hours before virus packaging, the cell culture medium was replaced with DMEM containing 1% fetal bovine serum, and the amount added was 6mL / 100mm culture dish;
[0122] (2) Add the plasmids to 500 μL opti-MEM medium according to Table 1, wherein the pWPXLd-CAR-eGFP plasmids are pwpxld-PD1.BBT2, pwpxld-Meso.28zT2, pwpxld-PSCA.28zT2, pwpxld-MUC1.28zT2, Either of pwpxld-HER2.28zT2 or pwpxld-GPC3.28zT2;
[0123] (3) Add 36 μg PEI to another 500 μL opti-MEM medium, mix well, and let stand at room temperature for 5 minutes;
[0124] (4) After mixing the plasmid and PEI, pipette and mix well, and let stand at room temperature for 25-30min;
[0125] (5) Add the mixed solution dropwise to the 293T cells, and the 293T cells are cultured in a 100mm culture dish;
[0126] (6) After 6 hours, the culture medium was r...
Embodiment 3
[0131]Example 3 Preparation of CAR-T cells
[0132] (1) Peripheral blood mononuclear cells (PBMC) were separated from whole blood using Ficoll density gradient centrifugation kit (GE Company);
[0133] (2) Using the Pan T cell magnetic bead sorting kit (Miltenyi Company) to separate T cells from PBMC;
[0134] (3) CD2 / CD3 / CD28 T cell activation expansion kit (Miltenyi Company) was used to activate T cells for 36 hours;
[0135] (4) After T cells were activated for 36 hours, discard the magnetic beads, resuspend T cells in 1640 medium containing 10% fetal bovine serum, 1000IU / mL IL2, add lentivirus according to Table 2, and add lentivirus every 10 6 Add 1mL lentivirus and 8μg / mL polybrene to each T cell, at 37℃, 5%CO 2 Infect twice in the incubator, each interval 8-12h;
[0136] (5) After virus infection, T cells were resuspended in 1640 medium containing 10% fetal bovine serum and 1000IU / mL IL-2, and every 10 6 Add 1mL lentivirus to each T cell, add fresh medium every 48h,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com