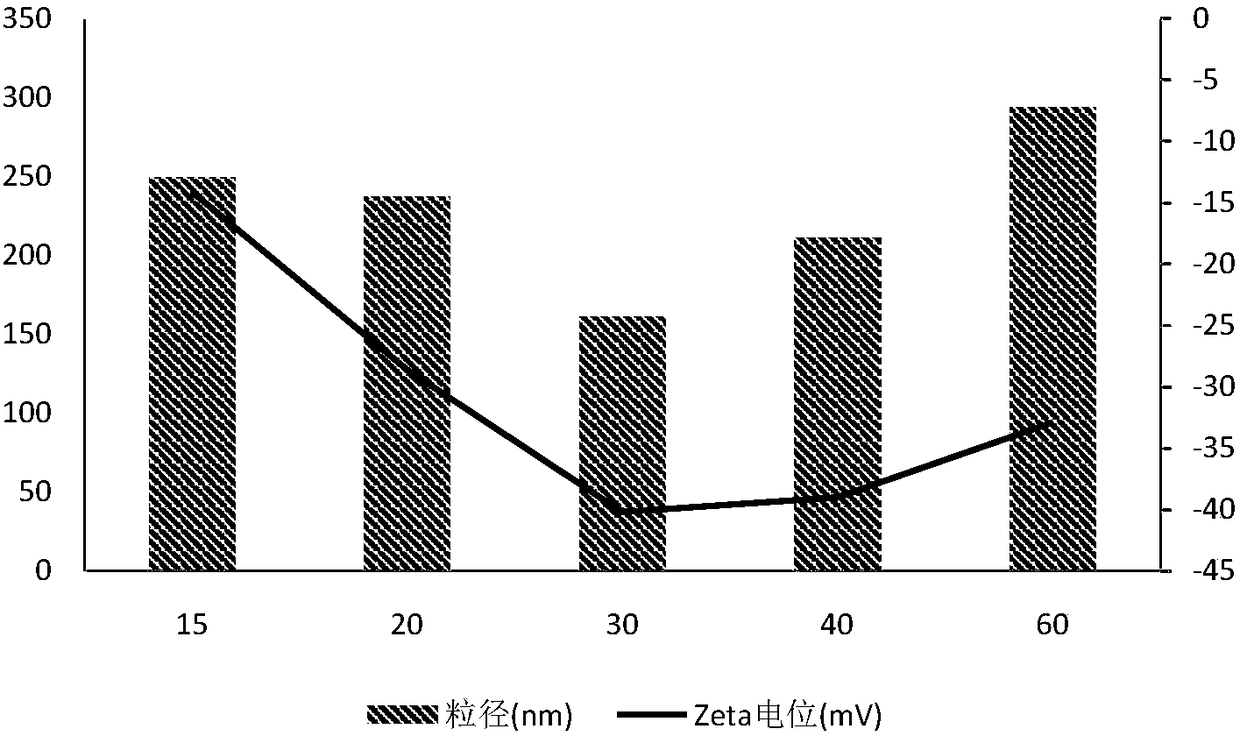
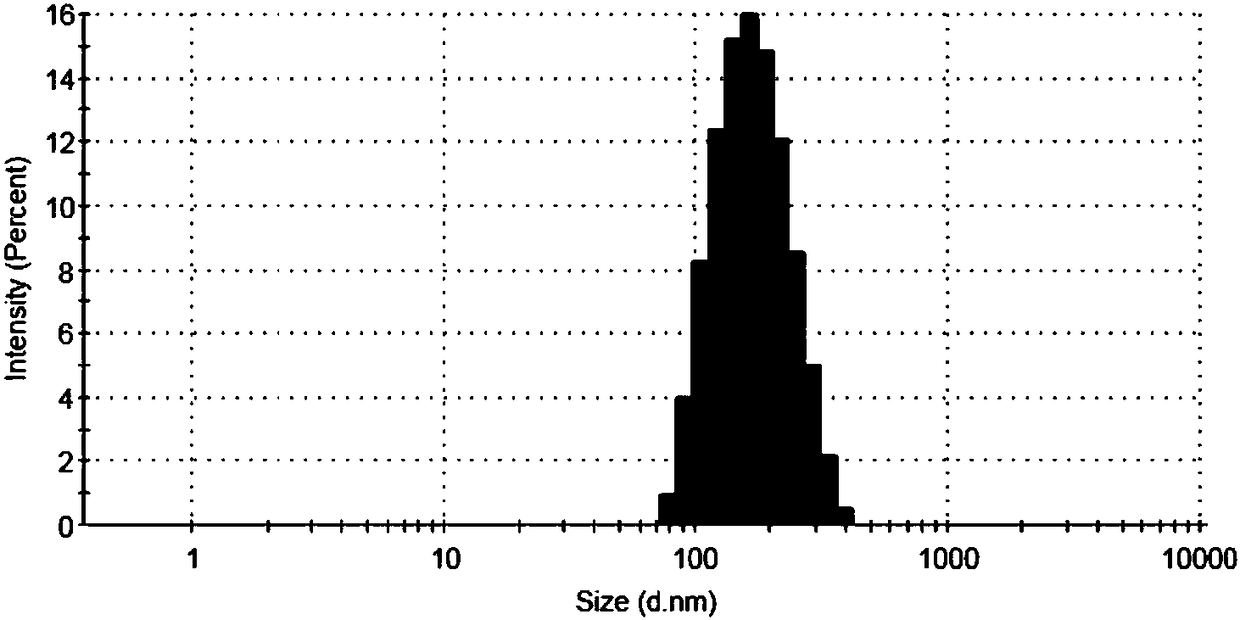
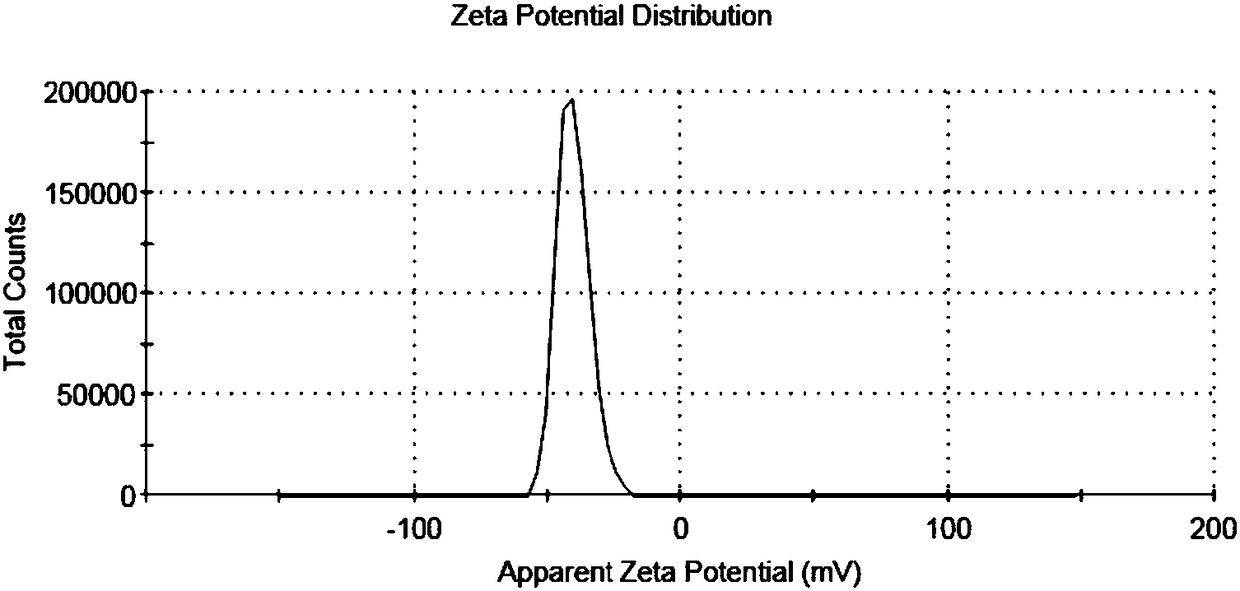
Polyacrylic acid-S-S-drug copolymer and preparation method thereof
A polyacrylic acid and copolymer technology, applied in the field of pharmaceutical preparations and polymer chemistry, can solve the problems of lack of pertinence, worsening side effects, and limited effects, and achieve good inhibitory effect, reduce toxic side effects, and small toxic side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 polyacrylic acid-S-S-gambogic acid copolymer (PAA-S-S-GA)
[0030] (1) Preparation method
[0031] 1. Dissolve 1.57g of gambogic acid in 50mL of dichloromethane, stir until completely dissolved, add 620mg of EDC and 438mg of HOBT under ice bath, and stir overnight at room temperature in the dark to obtain a bright yellow reaction solution, which is added to the reaction solution Add 1.69g of cystamine dihydrochloride, add 10mL of methanol to aid dissolution, adjust the pH to 7.4 with triethylamine, stir for 24h, and use NaHCO 3 After the aqueous solution was washed three times, the organic layer was dried by adding anhydrous sodium sulfate, filtered, and the dichloromethane was removed by rotary evaporation under reduced pressure, and dried in vacuo to obtain a yellow oily compound, which was a gambogic acid derivative, which was directly carried out to the next reaction.
[0032] m / z:763.3[M+H] + ; 1H NMR (CDCl 3 )δ8.64(s,1H), 6.71(d,1H), 6.58(d,1H), 6....
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com