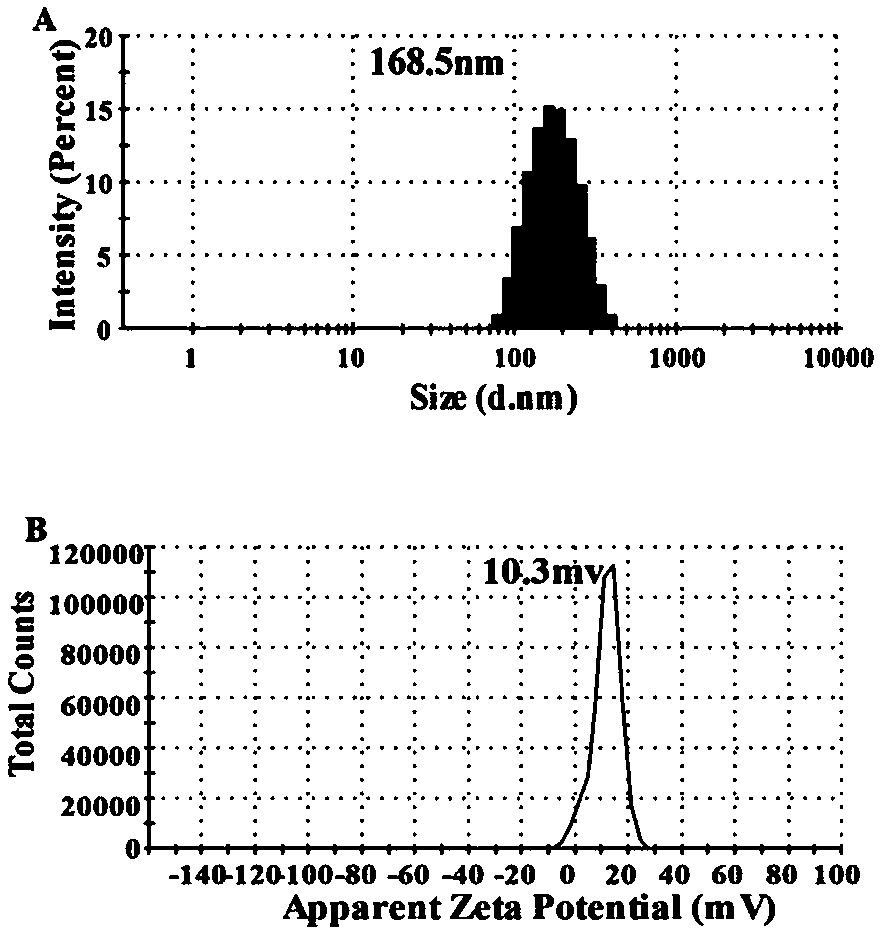
Preparation method of mesoporous silica-insulin nanometer slowly-released transdermal patch
A mesoporous silica and nano-sustained-release technology, applied in the field of pharmacy, can solve the problems of insulin physiological pain, transient hypoglycemia, and high price, and achieve the effects of avoiding transient hypoglycemia, high drug loading, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A: Dissolve the insulin raw material drug in an alkaline buffer solution with a pH of 7.47, and prepare a raw material drug solution with a concentration of 2 mg / mL for later use;
[0033] Weigh 0.6g CTAB, 10 mL ethylene glycol (EG), 90 mL H 2 O and 2.75 mL ammonia water (Ammonium Hydroxide, AMH), the above solutions were mixed, stirred in an oil bath at 60°C for 30 min, then quickly added 1.2 mL tetraethylsilane (TEOS), continued to stir for 2 h, then aged for 24 h, and the sample was taken out , centrifuged at 13000 rpm for 15 min, washed three times with ethanol and water respectively, then refluxed with 20 mL of acidic ethanol mixture for 24 h, centrifuged at 13000 rpm for 15 min, washed three times with ethanol and water respectively, and freeze-dried to obtain MSN-OH; Weigh 100 mg MSN-OH, ultrasonically dissolve it in 20 mL of absolute ethanol, then add 400 μL of 3-aminopropyltriethoxysilane (APTES), stir and react at room temperature for 24 h, centrifuge the nano...
Embodiment 2
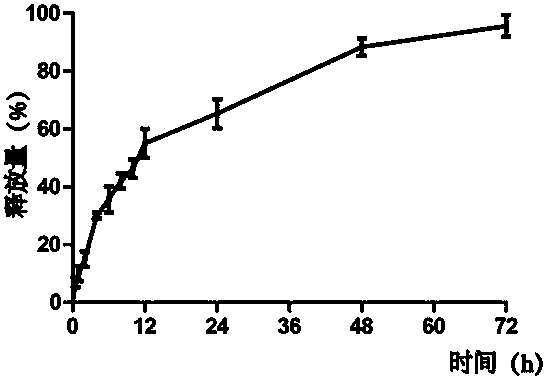
[0037] In vitro release of embodiment 2 ginsenoside / insulin nanogel
[0038] Place 1ml of the drug-loaded layer in a dialysis bag with a molecular weight cut-off of 10,000, and at the same time place the dialysis bag in 30 ml of PBS solution (NaCl, 137 mM; KCl, 2.7 mM, Na 2 HPO 4 ,10 mM; KH 2 PO 4 , 2 mM; pH 7.4), glucose was added to the buffer solution to achieve a higher blood glucose concentration in the human body, and then the PBS buffer solution was placed in a constant temperature shaker at 37°C with a speed of 233min / r. Take a sample of 500 μL, and at the same time add 500 μL of PBS with a higher blood glucose concentration. Use ultraviolet spectrophotometry to detect the wavelength of 276nm, and calculate the cumulative amount. Such as figure 2 As shown, reflecting the full release of insulin within 3 days.
Embodiment 3
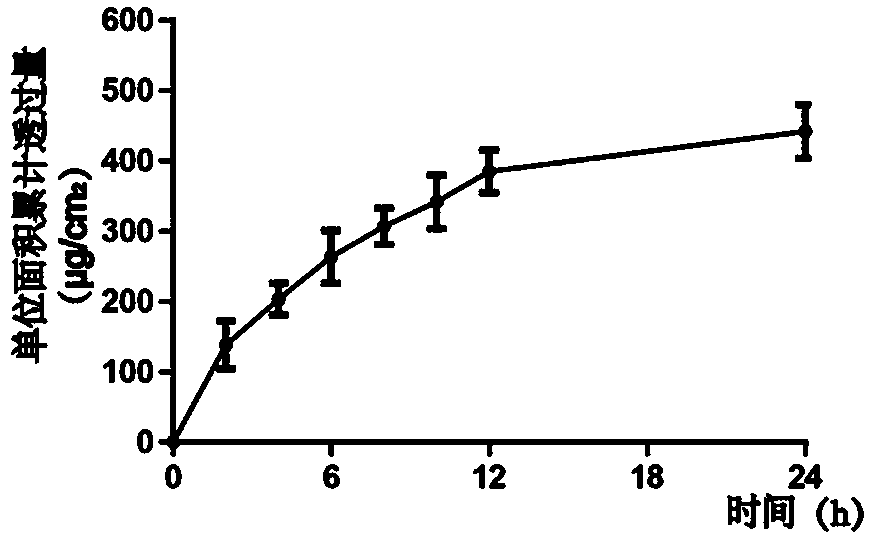
[0039] Example 3 In vitro percutaneous absorption experiment
[0040] Prepare 12 complete mouse abdomen skins, use the diffusion cell experiment, and calculate the penetration amount according to the formula
[0041] (Q, μg.cm 2 )
[0042]
[0043] In the formula, Cn is the drug concentration measured at the nth sampling point; Ci is the drug concentration measured at the ith sampling point; A is the effective contact area (cm 2 ); V is the receiving pool volume (ml); V0 is the sampling volume (ml).
[0044] Take the cumulative penetration per unit area (Q) as the ordinate, and time (t) as the abscissa to draw the transdermal absorption experiment, such as image 3 As shown, it shows that the mesoporous silica insulin nanogel has a high transdermal rate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com