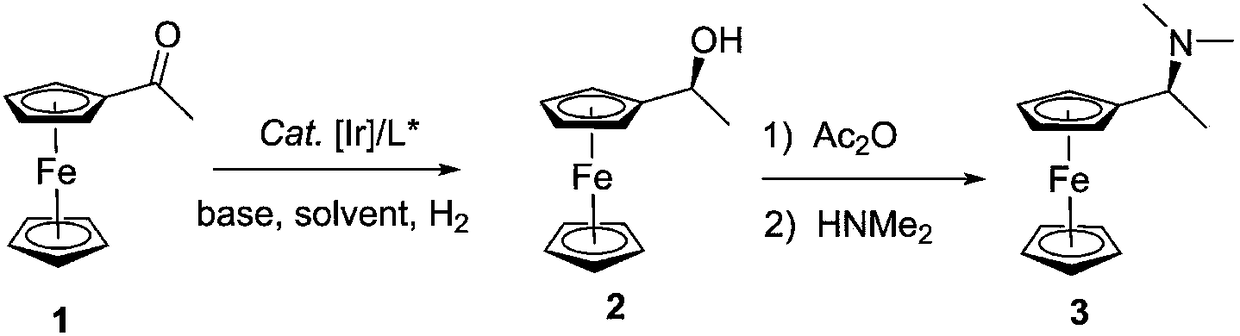
Process for preparing (S)-1-ferrocene ethyl dimethylamine
A kind of ferrocene ethyl dimethylamine, the technology of preparation process, applied in the direction of metallocene, organic compound/hydride/coordination complex catalyst, organic chemistry, etc., can solve the problem of large amount of catalyst and poor enantioselectivity control. High problems, to achieve the effect of low catalyst dosage, easy availability of raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
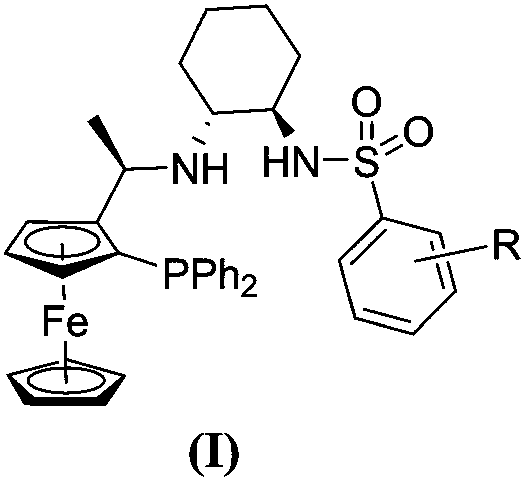
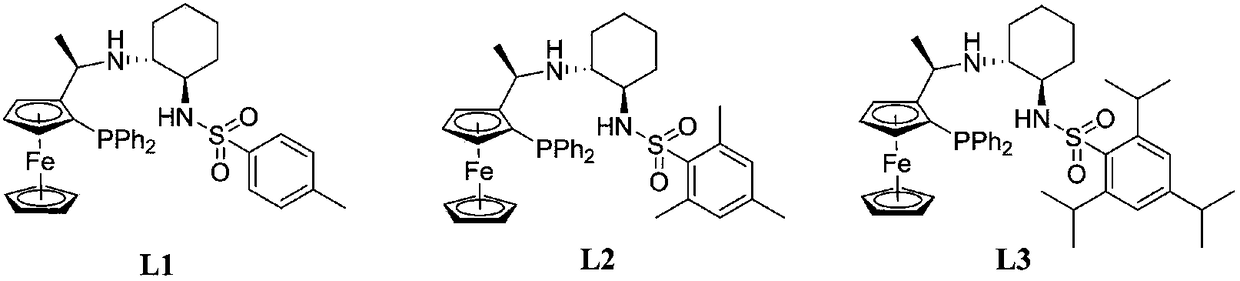
[0027] Example 1: Synthesis of Ligand L1
[0028]
[0029] Compound 4 (4.41 g, 10 mmol) was dissolved in 8.0 mL of acetic anhydride and reacted at 55° C. for 4 hours. After the reaction was completed, excess acetic anhydride was recovered under reduced pressure, and then impurities with low boiling points were removed under high vacuum to obtain crude product 5, which was directly used in the next step without purification.
[0030]
[0031] Crude products 5 (0.46g, 0.1mmol) and 6 (0.54g, 0.2mmol) were added to the reaction flask, and after nitrogen was replaced, 5mL of methanol was added and reacted at 50°C for 12 hours. Concentration and column chromatography gave yellow ligand L1 (0.41 g, 61%).
[0032]
[0033] 1 H NMR (600MHz, CDCl 3 )δ7.70(d, J=8.2Hz, 2H), 7.50–7.44(m, 2H), 7.39–7.34(m, 3H), 7.24(d, J=8.1Hz, 2H), 7.18–7.12(m ,1H),7.09–7.01(m,4H),5.89(s,1H),4.49(s,1H),4.34(t,J=2.4Hz,1H),4.05(s,5H),4.03–3.96( m,1H),3.68(s,1H),2.42(s,3H),2.10(s,2H),1.97–1.87(m...
Embodiment 2
[0034] Example 2: Synthesis of Ligand L2
[0035] The preparation method of crude product 5 is the same as that of Example 1.
[0036]
[0037] Crude products 5 (0.46 g, 0.1 mmol) and 7 (0.60 g, 0.2 mmol) were added to the reaction flask, and after replacing the nitrogen, 5 mL of methanol was added and reacted at 50° C. for 12 hours. Concentration and column chromatography gave yellow ligand L2 (0.37 g, 53%).
[0038]
[0039] 1 H NMR (600MHz, CDCl 3)δ7.52–7.46(m,2H),7.39–7.34(m,3H),7.14(m,1H),7.01(s,4H),6.91(s,2H),5.92(s,1H),4.48 (s,1H),4.35–4.31(s,1H),4.05(m,5H),4.03(s,1H),3.69(s,1H),2.59(s,6H),2.31(s,3H), 2.17–2.01(m,2H),1.92–1.84(m,1H),1.75(s,1H),1.43(m,5H),1.27(m,1H),0.96(m,2H),0.81–0.72( m,1H),-0.44(d,J=8.1Hz,1H). 13 CNMR (151MHz, CDCl 3 )δ141.45,140.03,139.95,138.98,136.43,136.36,135.03,134.86,134.19,132.59,132.44,131.68,129.14,128.31,128.26,128.12,128.06,74.05,74.00,71.03,71.00,69.62,69.31,69.28,69.14 ,57.66,56.89,46.18,31.93,29.70,24.78,23.87,23.03,20.86...
Embodiment 4
[0046] Embodiment 4: Preparation of (S)-1-ferroceneethyl dimethylamine
[0047] (1) Ligand L1 (0.32g, 0.48mmol), metal [Ir(COD)Cl] 2 (0.16g, 0.22mmol) was added to a reaction flask, methanol (20mL) was added under an argon atmosphere, and the reaction was stirred at 25°C for 0.5h to obtain a catalyst.
[0048] (2) Add acetylated ferrocene 1 (1.03kg, 4.52mol) in the autoclave, add the catalyst prepared by step (1), sodium methoxide (1.19g, 22mmol), methanol (1.50L), and fill with H 2 (3.0MPa), reacted at 40°C for 12h, concentrated under reduced pressure to recover the organic solvent, and obtained (S)-1-ferrocenyl ethanol 2 (1.03kg, 4.47mol), yield: 99%, purity: 98%, The ee value was 91%.
[0049] (3) Put (S)-1-ferrocenyl ethanol 2 (1.03kg, 4.47mol), dichloromethane (3.0L), and triethylamine (902.9g, 8.94mol) prepared above into the reactor in sequence Add acetic anhydride (683.4g, 6.70mol) dropwise at room temperature, continue to stir for 3 hours; then slowly add 4.31kg of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com