Human T lymphocytes carrying cd20/cd19 bispecific chimeric antigen receptor and preparation method and application
A chimeric antigen receptor and bispecific technology, applied in the field of human T lymphocytes and preparation, can solve the problems of no significant increase in lentiviral transduction efficiency, time-consuming and laborious, and cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Construction of lentiviral expression vector plvx-EF1a-CD20CD19CAR
[0052] The amino acid sequences of each part of the second-generation bispecific chimeric antigen receptor are connected to form the amino acid sequence of the chimeric antigen receptor, as shown in SEQ ID No.2, and the nucleic acid sequence of the chimeric antigen receptor is optimized , the nucleic acid sequence of the chimeric antigen receptor is shown in SEQ ID No.3, after introducing restriction sites SpeI and BamHI, artificial whole gene synthesis is performed, and cloned into a lentiviral vector to obtain plvx-EF1a-CD20CD19CAR recombinant lentivirus The expression vector plasmid, the recombinant plasmid was sent to Sangon Bioengineering Company for sequencing, and the sequencing results were compared with the fitted sequence and confirmed to be completely correct.
Embodiment 2
[0053] Example 2: Peripheral Blood Mononuclear Cells (PBMC) Isolation and Culture
[0054] Before separating PBMCs, in a biosafety cabinet in a GMP laboratory, coat a 6-well plate with anti-human CD3 monoclonal antibody and human fibronectin fragments, and take anti-human CD3 monoclonal antibody (OKT-3, Acrobiosystem ) 30ul and 500ug / ml human fibronectin fragment (Novoprotein) 600ul, diluted to 6ml with PBS, mixed well and added to each well, 1ml per well, sealed the plate with a sealing film, and placed at 4 degrees Refrigerate overnight.
[0055] In the biosafety cabinet of the GMP laboratory, separate PBMCs from 60ml of peripheral blood of healthy donors with human lymphocyte separation medium Ficollpaque plus (GE), resuspend with lymphocyte medium, place them in T150 culture flasks, and incubate at 37 degrees for 2h , retain unattached cells, count the cells and resuspend them in serum-free lymphocyte medium, take half of the cells and divide them into each well of a six-...
Embodiment 3
[0059] Embodiment 3: CART cell preparation
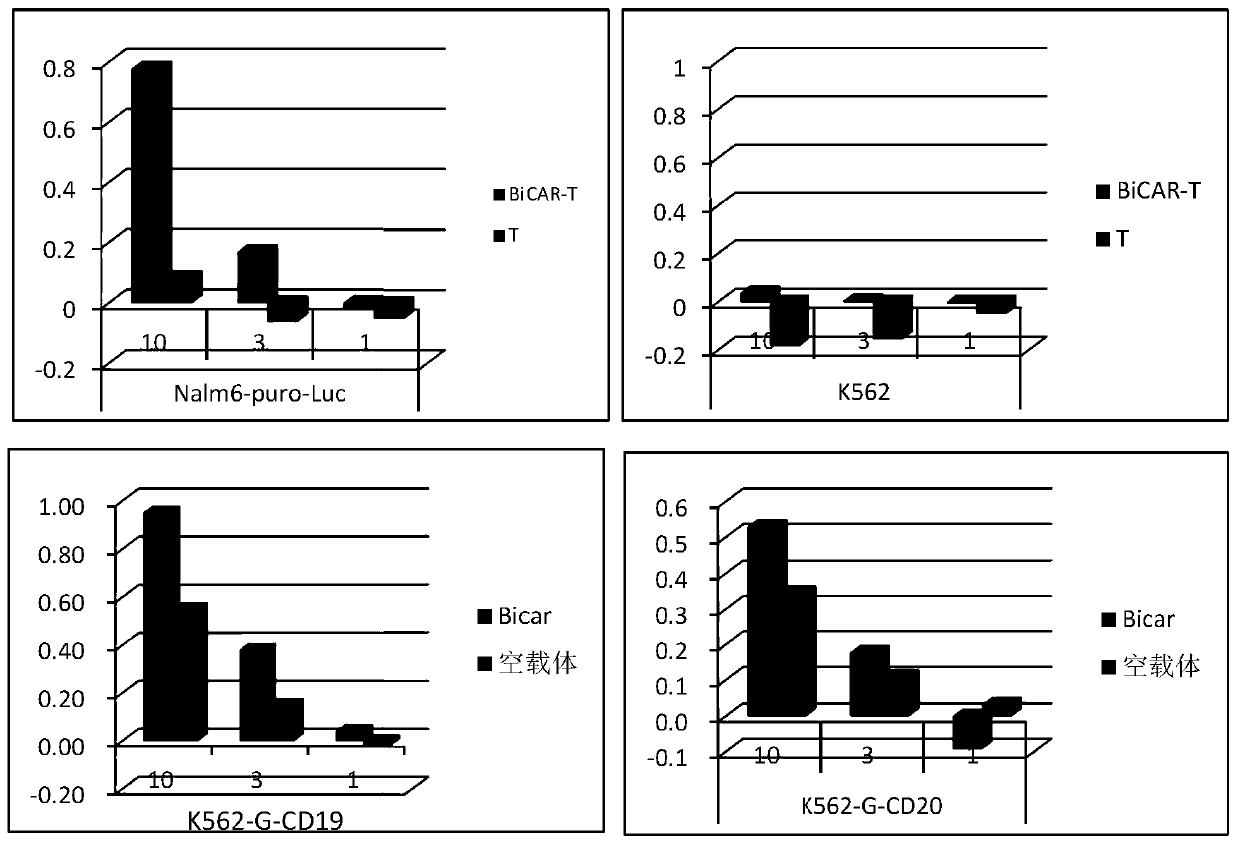
[0060] Take CD3+Fibronectin (fibronectin fragment) activated lymphocytes for 48h 2×10 6 For each, add 40ul of virus solution and 0.5ul of DEAE-dextran, incubate at 37°C for 10min, then add AIM-V medium (ie, serum-free medium) (containing 10% autologous plasma, 300U / ml hIL-2 and 10ng / ml hIL-15) 400ul, incubate at 37°C for 3.5h, then replace with fresh medium AIM-V (containing 10% autologous plasma, 300U / ml hIL-2 and 10ng / ml hIL-15), adjust the cell density to 1× 10 6 cells / ml, and then replenish or replace the medium every 2-3 days to keep the cell density at (1-3)×10 6 pieces / ml.
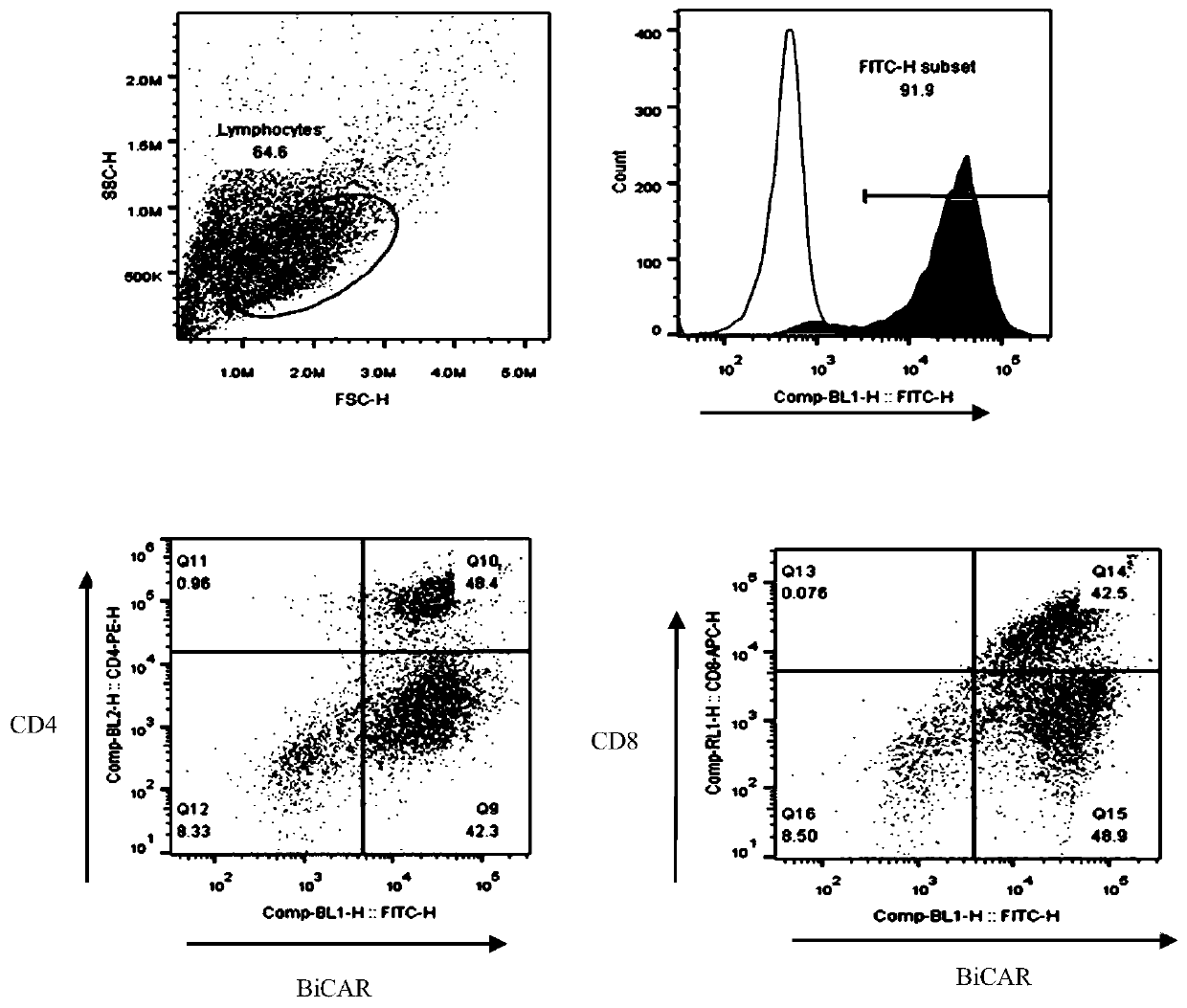
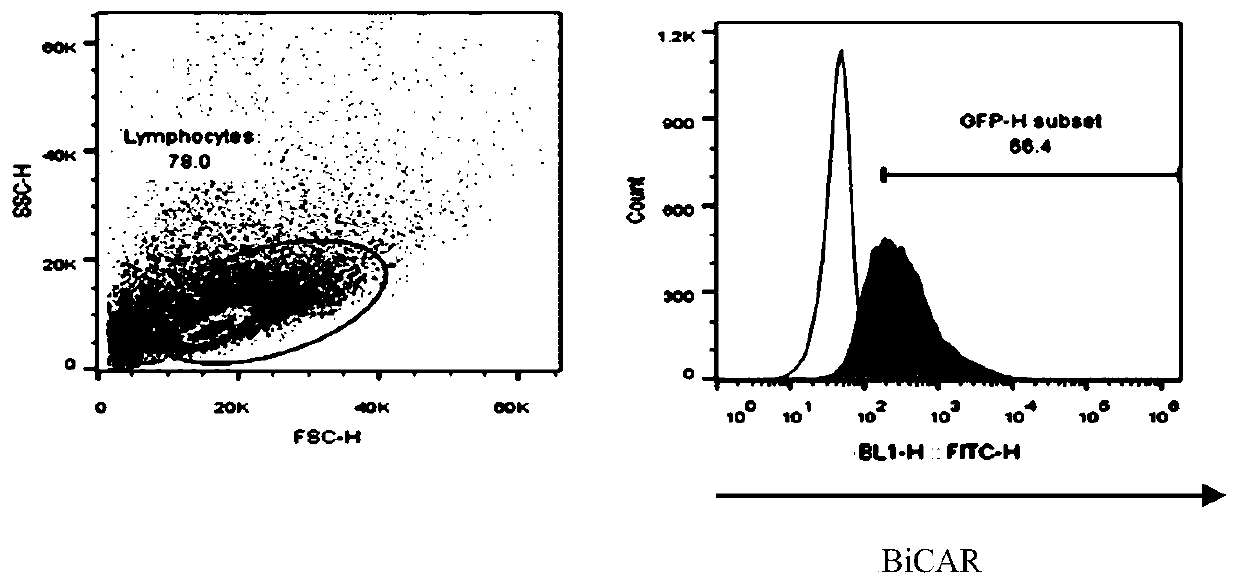
[0061] On the 8th day of culture, the positive rate of CAR+ and the positive rate of CAR in CD4+ and CD8+ T lymphocytes were detected by flow cytometry. The results are summarized in Table 2. For the results of flow cytometry, please refer to image 3 ,pass image 3 It can be seen that CAR+ (%) is 91.9; CD4+CAR+ (%) is 48.4%, and CD8+CAR+ (%) is 42.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com