Inactivated vaccine prepared by Newcastle disease virus heat stable strain and preparation method thereof
A technology of Newcastle disease virus and inactivated vaccines, which is applied in the field of biological product manufacturing, can solve the problems such as long time for formaldehyde inactivation, and achieve the effect of overcoming relatively poor thermal stability, broad application prospects, and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment one: the preparation of the inactivated vaccine of Newcastle disease virus thermostable strain
[0030] 1. Preparation of viral allantoic fluid
[0031] Dilute the allantoic fluid of a stable strain of Newcastle disease virus 10 times, shake well, inoculate 9-11-day-old SPF chicken embryos through the allantoic cavity, inoculate 0.1mL per embryo, and continue to incubate for 5 days in an incubator at 37°C. Harvest The allantoic fluid was stored in a sterile container and stored at -20°C after sampling.
[0032] 2. Dilution of viral allantoic fluid
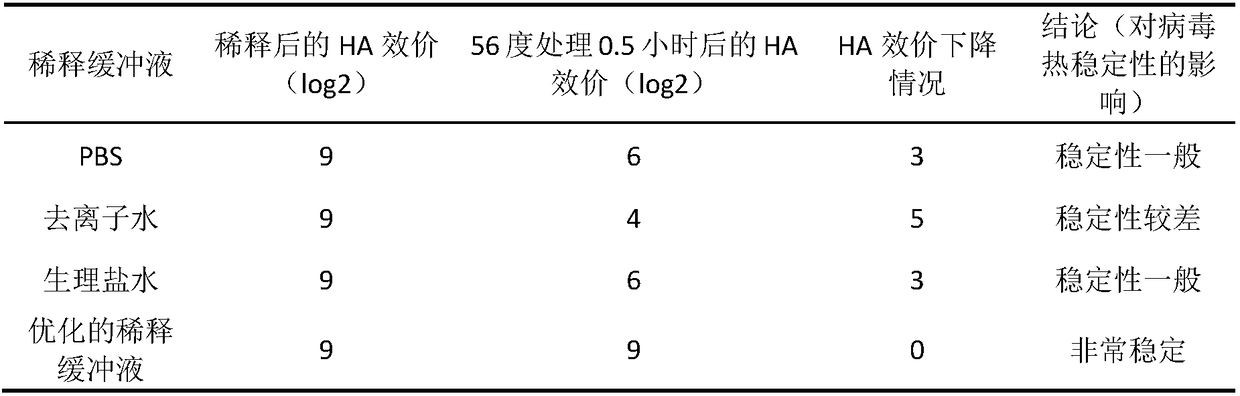
[0033] Prior to virus inactivation, virus quantification is required on the collected allantoic fluid diluted to 10 with the optimized dilution buffer. 8.0 EID 50 / ml. In this embodiment, the optimized dilution buffer refers to 100mmol / L Tris-HCl (pH 7.8), 5mmol / L sodium chloride, 5mmol / L potassium chloride, 2.0mmol / L calcium chloride, 1.0mmol / L Diluent consisting of L magnesium chloride and 1mmol / L disodium ...
Embodiment 2
[0063] Embodiment two: the shelf life test of vaccine
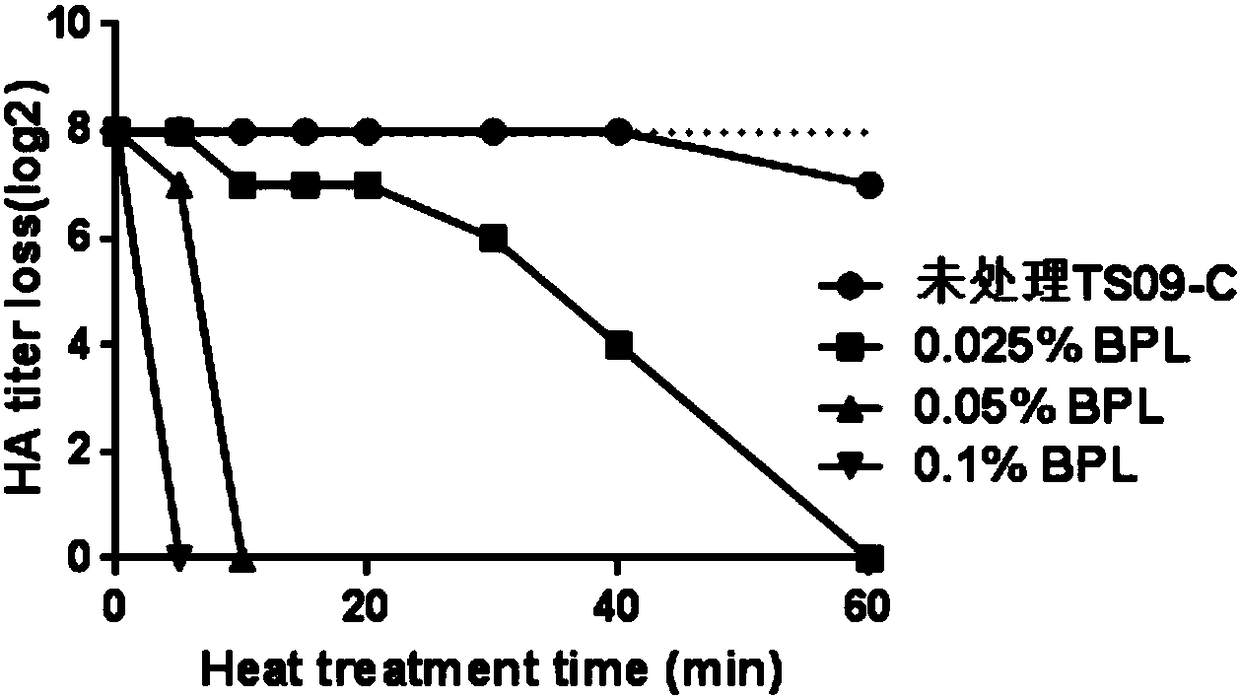
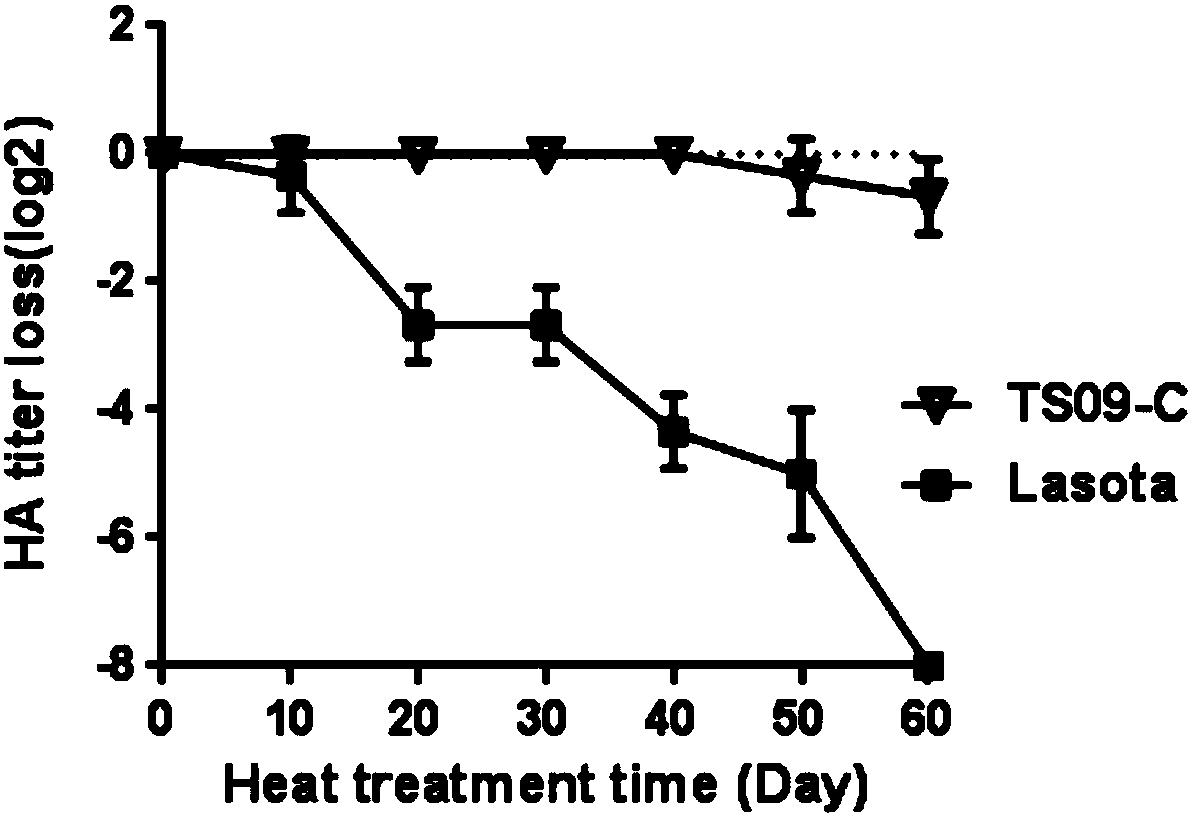
[0064] The prepared inactivated vaccine was placed in a 42°C incubator, and samples were taken every 10 days to detect the titer of HA. At the same time, the LaSota standard strain that was inactivated by the same method was set as the control group, and three replicates were set for each sample. Such as figure 2 As shown, the results show that the prepared inactivated vaccine still maintains good thermal stability at 42°C, and the hemagglutination titer of the virus does not change significantly after being placed for 60 days, while the hemagglutination titer of the control group LaSota has changed from the initial 2 8 down to 20. It shows that the prepared inactivated vaccine has good thermal stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com