Application of myricetin to preparation of medicine for inhibiting human immunodeficiency virus infection
A technology related to human immunodeficiency and virus infection, which is applied in the field of medicine, can solve the problems of interfering with IN-LEDGF/p75 interaction and unsuccessful marketing, and achieve good inhibitory activity and high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
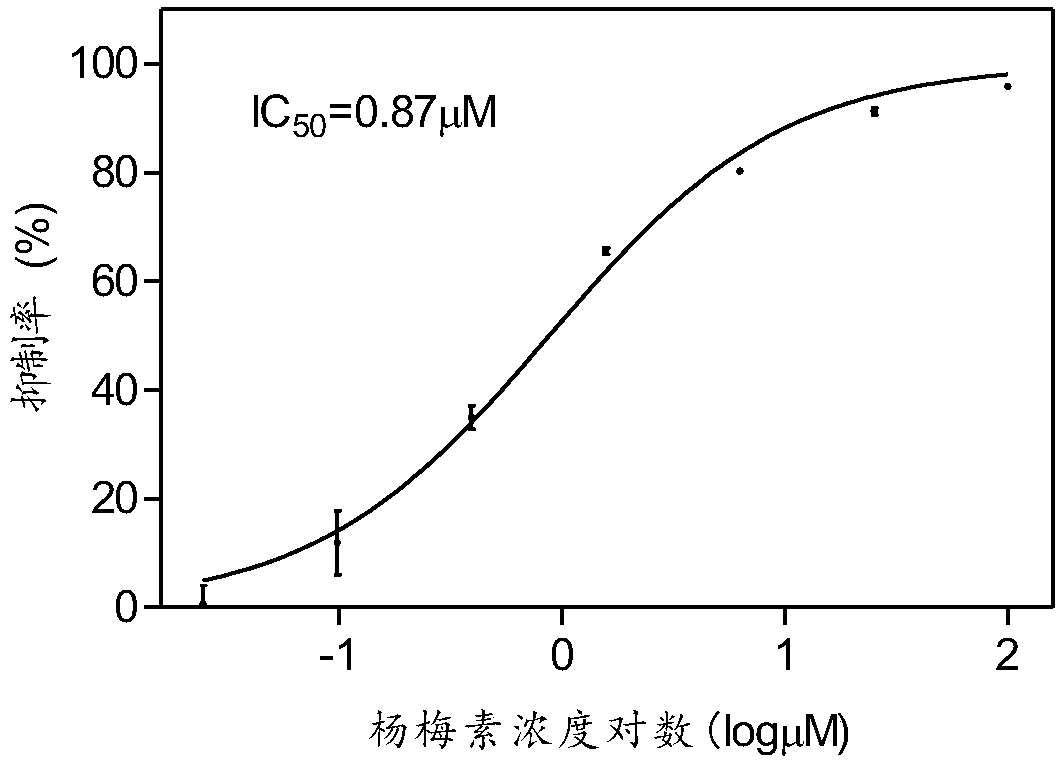
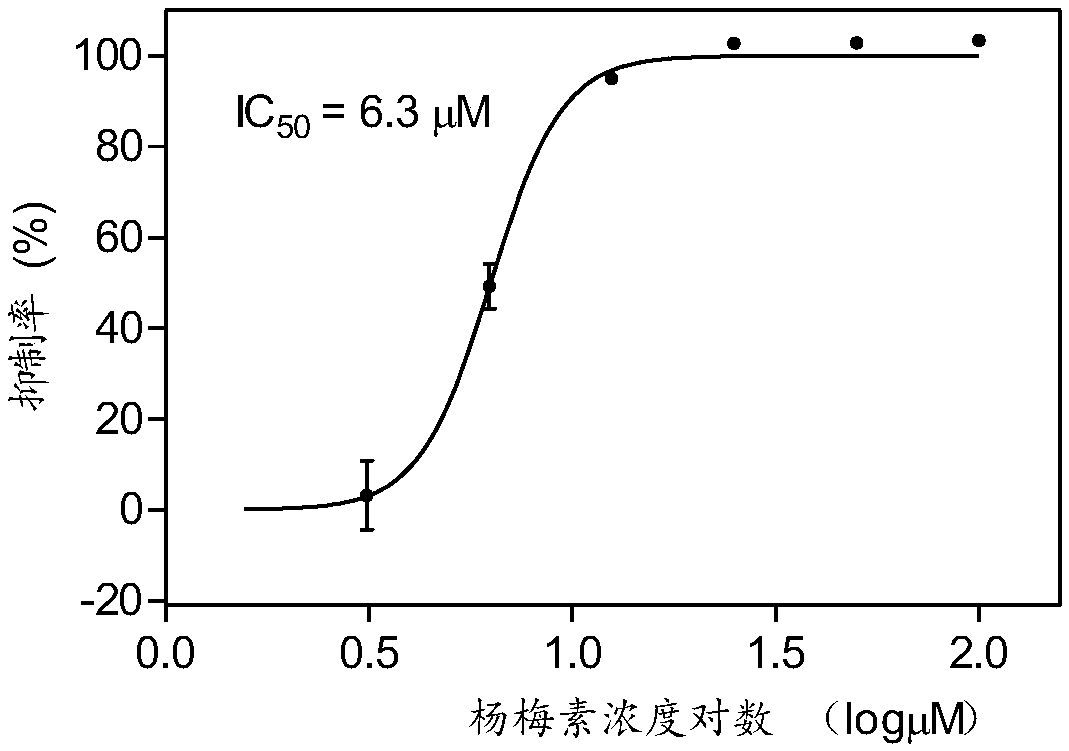
[0031] Example 2: Determination of activity of compound on integrase enzyme inhibition
[0032] 1. Experimental principle: IN can cut target DNA and integrate its donor DNA into target DNA. Based on this, digoxin was labeled at the 3 end of the target DNA, and biotin was labeled at the 5' end of the donor DNA. Since IN is able to cleave the target DNA, it integrates its donor DNA into the target DNA. Thus, a 5'biotin-3'digoxigenin double labeled DNA product will be formed. Streptavidin magnetic beads (SA-MB) are added, and the DNA product is captured on the surface of the magnetic beads through the specific reaction of 5' biotin and streptavidin. Then add alkaline phosphatase-labeled digoxin antibody (AP-anti-DIG AB), and connect alkaline phosphatase (AP) to the magnetic beads through the immune reaction between the antibody and digoxin. AP was finally detected by ELISA to detect the integration reaction catalyzed by IN.
[0033]2. Experimental procedure: the reaction was ...
Embodiment 3
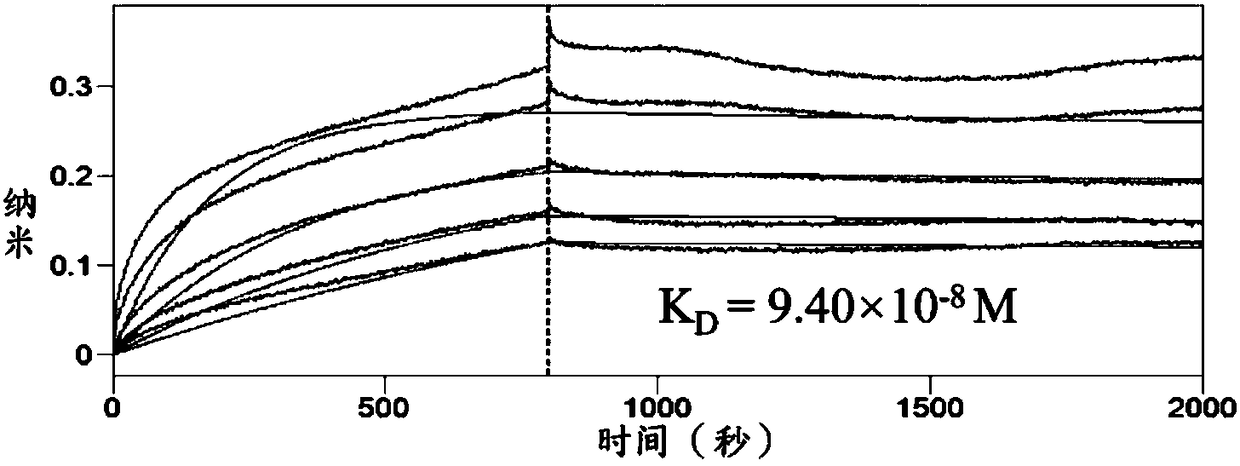
[0035] Example 3. Determination of the binding affinity between the compound and integrase.
[0036] 1. Experimental principle: The bottom of the biosensor made of optical fiber is covered with a biomolecular compatibility layer, and after coupling with IN, a biofilm layer is formed, and then the ligand is used to react with small molecules. When a small molecule binds to the ligand coupled at the bottom of the sensor, the thickness of the biofilm layer will increase, and the reflected light interference spectrum curve will shift. The instrument can monitor the change of the phase shift in real time and convert it into a measurable intermolecular interaction. parameters.
[0037] 2. Experimental procedure: Bio-Layer Interferometry (BLI) was completed in Octet RED96 system. A 96-well plate matching the instrument (Fluotrace 600, LOT E110103S) was used in the experiment, and the reaction volume was 200 μl. The biotinylated IN was immobilized on the streptavidin-coated probe an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com