A kind of tablet of bepotastine besilate and preparation method thereof
A technology of bepotastine bepotastine and tablets, which is applied in the field of tablets and preparations of bepotastine bepotastine, can solve the problems of limited production capacity, high equipment requirements, high equipment cost, etc., and achieve tablet weight Stable, low safety risk, smooth surface effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
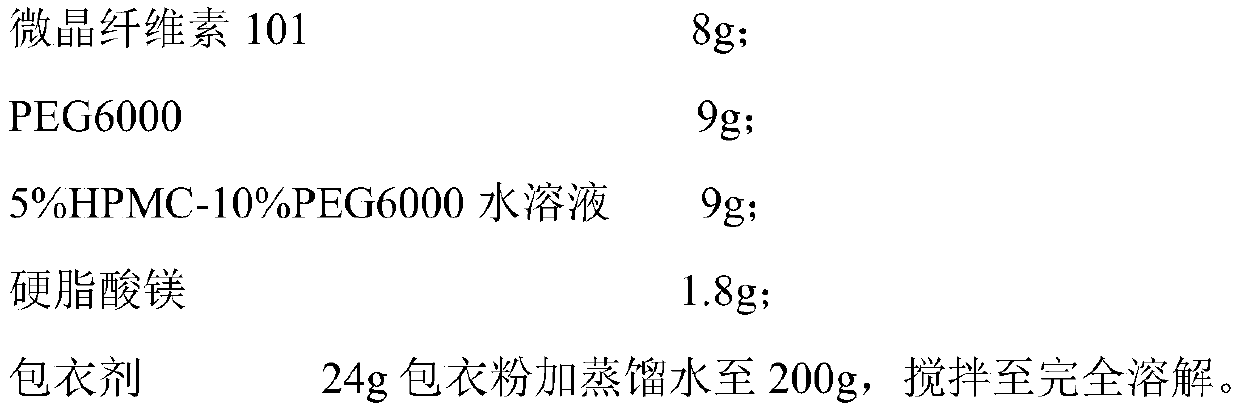
[0034] Tablet prescription (1000 formulation units):
[0035]
[0036]
[0037] The preparation method of the present invention comprises the following steps:
[0038] (1) Pretreatment: sieve the active ingredients and auxiliary materials for later use;
[0039] (2) Premixing: mixing the active ingredient with the filler and PEG6000;
[0040] (3) Granulation: add binder solution to make soft material, 20-30 mesh granulation;
[0041] (4) Drying and sizing: drying the granules obtained in step (3) at 50°C until the water content of the granules is no higher than 3%, and then making 20-30 mesh granules;
[0042] (5) Total blending: add anti-sticking agent and lubricant to the granulated granules, and detect the intermediate after mixing;
[0043] (6) Tablet compression: According to the results of intermediate detection, calculate the weight of the tablet, press the tablet to make a tablet core, and control the tablet hardness to 40-80N;
[0044] (7) Coating: the table...
Embodiment 2
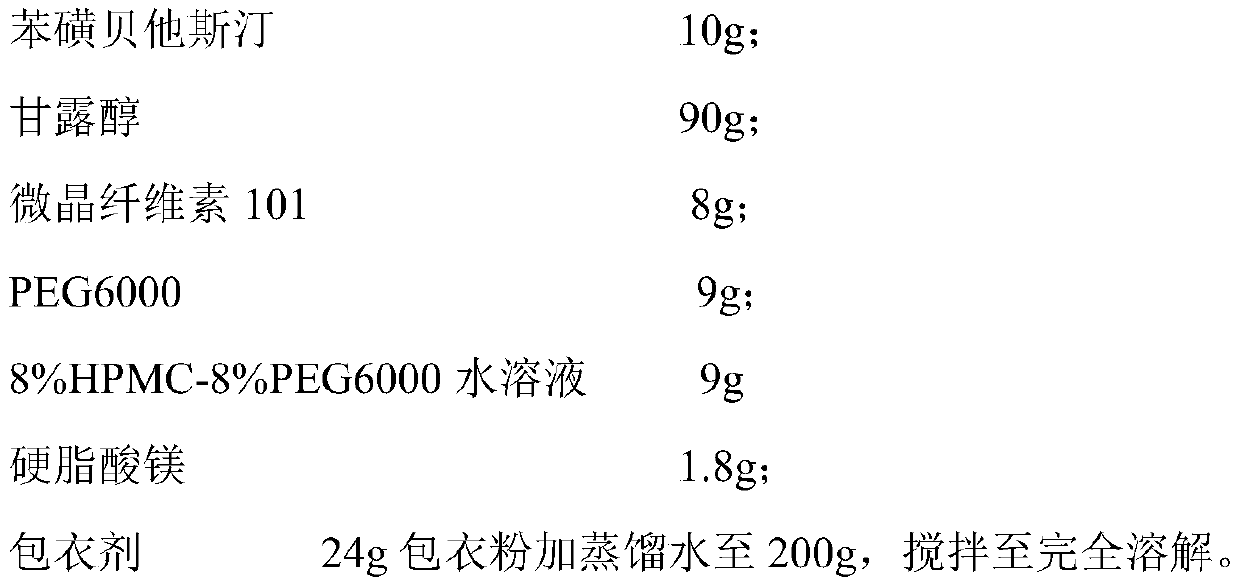
[0046] Tablet prescription (1000 formulation units):
[0047]
[0048] The preparation method of the present invention comprises the following steps:
[0049] (1) Pretreatment: sieve the active ingredients and auxiliary materials for later use;
[0050] (2) Premixing: mixing the active ingredient with the filler and PEG6000;
[0051] (3) Granulation: add binder solution to make soft material, 20-30 mesh granulation;
[0052] (4) Drying and sizing: drying the granules obtained in step (3) at 50°C until the water content of the granules is no higher than 3%, and then making 20-30 mesh granules;
[0053] (5) Total blending: add anti-sticking agent and lubricant to the granulated granules, and detect the intermediate after mixing;
[0054] (6) Tablet compression: according to the results of intermediate detection, calculate the weight of the tablet, press the tablet to make a tablet core, and control the tablet hardness to 40-80N;
[0055] (7) Coating: the tablet core is co...
Embodiment 3
[0104] Embodiment 3: Effect verification (each inspection item can be carried out with reference to the fourth part of the Chinese Pharmacopoeia of the 2015 edition)
[0105] 1. Test the fluidity and compressibility of the intermediate particles of the examples and comparative examples, and the specific results are shown in Table 1
[0106] Table 1 Example sample intermediate particle fluidity, compressibility and hygroscopicity testing results
[0107]
[0108] 2, detect the content uniformity (general rule 0941) of embodiment and comparative example sample, concrete result is shown in Table 2
[0109] Table 2 embodiment sample content uniformity detection result
[0110]
[0111]
[0112] 3. The friability (general rule 0941) and hardness of samples (plain slices) of detection examples and comparative examples, the specific results are shown in Table 3
[0113] Table 3 embodiment sample friability and hardness testing results
[0114] sample Friability...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com