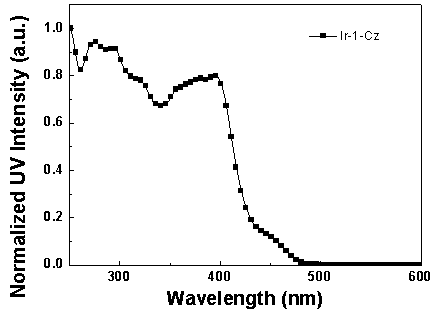
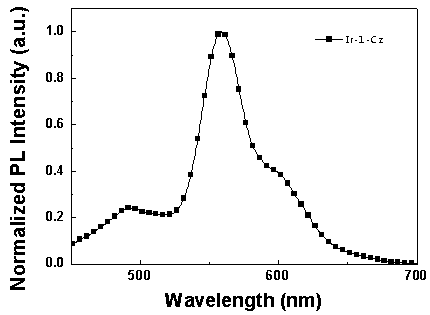
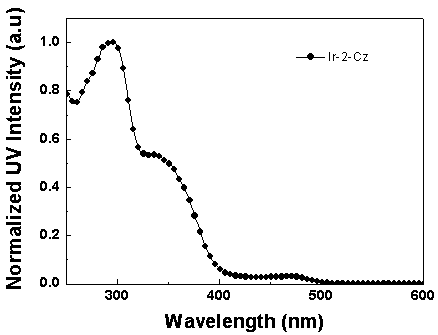
Iridium complex luminescent material containing dibenzothiophene sulfone group and its application
A technology of iridium complexes and thiophene sulfone groups, which is applied in the field of organic electroluminescent materials to achieve the effect of improving electron injection and transport capabilities and improving luminescent properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 2-(pyridin-2-yl)-7-(9-n-butylcarbazol-3 base)dibenzothiophene sulfone:
[0033]
[0034] (1) Synthesis of dibenzothiophene sulfone:
[0035]
[0036] Add 3.7g (20.0mmol) of dibenzothiophene, 10mL of glacial acetic acid and 6mL of 30% hydrogen peroxide into a 50mL round bottom flask, heat to 90°C, react for 0.5h, then add 2mL of 30% hydrogen peroxide, and continue the reaction for 0.5h . Cool to room temperature, filter with suction, wash with plenty of water, and dry in vacuum. Chloroform was recrystallized to obtain 4.1 g of white solid with a yield of 96%. 1 H NMR (400MHz, CDCl 3 , TMS) δ (ppm): 7.96 (d, J = 8.6Hz, 2H), 7.65 (d, J = 9.8Hz, 2H), 7.39 ~ 7.33 (m, 4H).
[0037] (2) Synthesis of 2,7-dibromodibenzothiophene sulfone:
[0038]
[0039] Add 3.89g (18.0mmol) dibenzothiophene sulfone and 120mL concentrated sulfuric acid into the flask, and stir to dissolve it. Add 4.0 g of NBS in small portions, and react at room temperature for 1 hou...
Embodiment 2
[0047] Synthesis of 3-(pyridin-2-yl)-6-(9-n-butylcarbazol-3 base) dibenzothiophene sulfone:
[0048]
[0049] (1) Synthesis of 3,6-dibromodibenzothiophene:
[0050]
[0051] Add 9.2g (50.0mmol) dibenzothiophene and 100mL chloroform into a 250mL three-necked flask, add 7.7mL (150mmol) liquid bromine dropwise at 0-5°C, and react at room temperature for 40h. Add saturated NaHSO 3 The excess liquid bromine was removed by aqueous solution to obtain a pale yellow solid, which was washed with water and ethanol to a white solid. Vacuum drying afforded 11.5 g of white solid, yield 67%. 1 HNMR (400MHz, CDCl 3 , TMS) δ (ppm): 8.22 (s, 2H), 7.71~7.69 (d, J=8.4Hz, 2H), 7.58~7.56 (dd, J=10.4Hz, 2H).
[0052] (2) Synthesis of 3,6-dibromodibenzothiophene sulfone:
[0053]
[0054] 6.8g (20.0mmol) 3,6-dibromodibenzothiophene, 150mL glacial acetic acid and 120mL tetrahydrofuran, and 15mL H 2 o 2 Add it to a two-neck flask, heat to 120°C, and react for 6h. After cooling to room ...
Embodiment 3
[0062] Synthesis of iridium complexes Ir-1-Cz and Ir-2-Cz:
[0063]
[0064] (1) Bis(2-(pyridin-2-yl)-7-(9-n-butylcarbazol-3yl)dibenzothiophene sulfone-N,C 2 ) (picolinic acid) iridium (Ⅲ) [Ir-1-Cz] synthesis.
[0065] Add 386.1mg (0.75mmol) of 2-(pyridin-2-yl)-7-(9-n-butylcarbazol-3yl)dibenzothiophene sulfone, 45mL of ethylene glycol monoethyl ether and 15mL of water into a 100mL In the three-neck flask, add 120.1mgIrCl rapidly under the protection of argon 3 ·3H 2 O, 100 ℃ constant temperature reaction 20h. After cooling, a yellow solid was produced, which was filtered by suction, washed with water and a little ethanol successively, and dried in vacuum to obtain a yellow powder. The product was directly used in the next reaction without further separation and purification.
[0066] In a 50 mL three-necked flask, 285.7 mg (0.082 mmol) of the reaction product of the previous step, 47.5 mg of 2-pyridinecarboxylic acid, 102 mg of sodium carbonate and 35 mL of ethylene gl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminous efficiency | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com