Quaternary phosphonium salts with p-toluenesulfonate as anions as well as synthesis method, preparation method and application of quaternary phosphonium salts
A technology of p-toluenesulfonate and synthesis method, which is applied in the direction of sulfonate preparation, chemical instruments and methods, and active ingredients of phosphorus compounds, etc. It can solve the problems of high molecular weight, harsh synthesis conditions and routes, and influence on the use effect of quaternary phosphonium compounds. , to achieve the effect of less chemical reagents, high yield and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
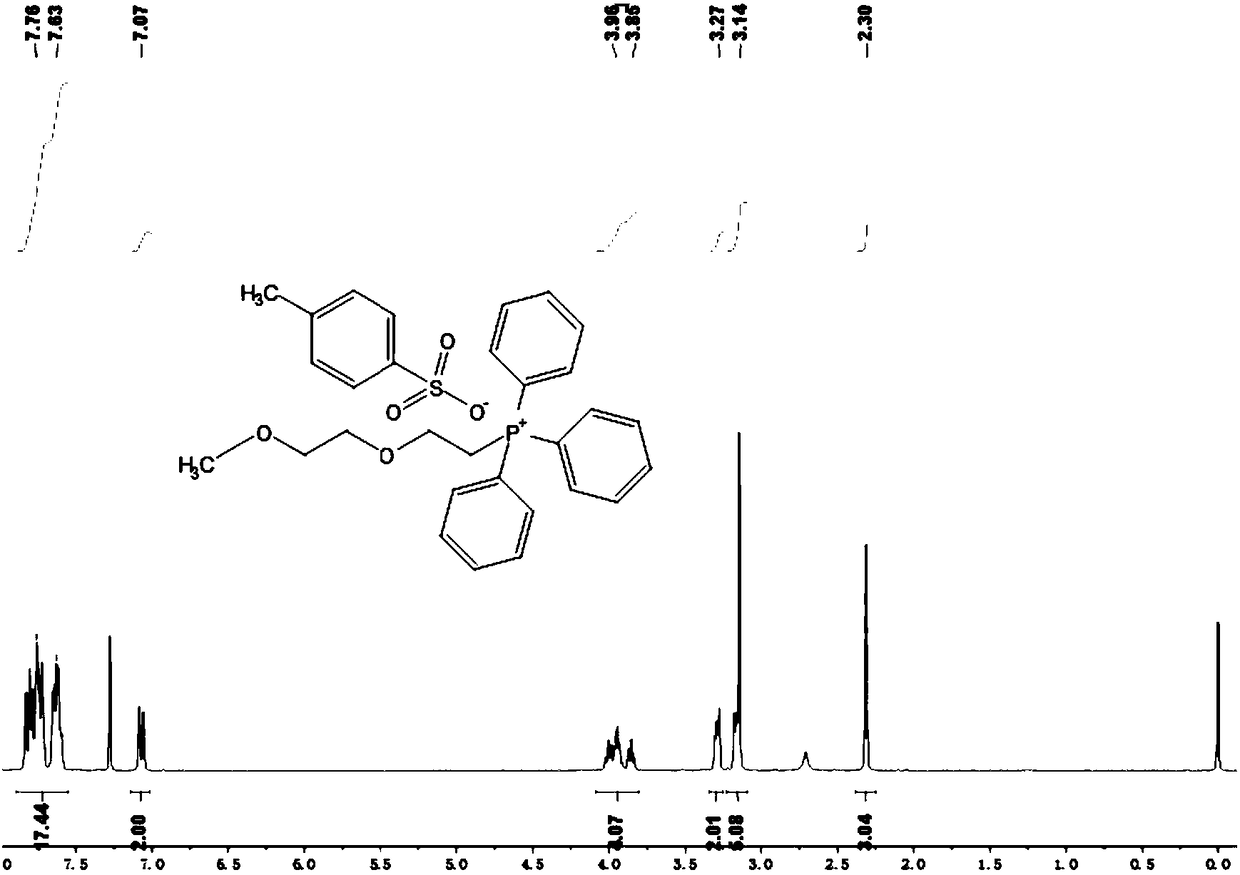
[0096] Embodiment 1: Preparation of diethylene glycol monomethyl ether triphenyl-p-toluenesulfonate
[0097] Dissolve 12.2mmol of diethylene glycol monomethyl ether compound in 15ml of THF, then add 18.3mmol of p-toluenesulfonyl chloride under stirring conditions, and finally add 16.07mmol of p-toluenesulfonyl chloride dropwise slowly within 1 hour under stirring conditions in an ice-water bath. mol of KOH aqueous solution 2.5ml; remove the ice-water bath, return to room temperature and stir for 7 hours; pour the resulting suspension into 10ml of CH 2 Cl 2 and 5ml of ice water, the aqueous layer was washed with CH 2 Cl 2 extraction; then the organic phases were combined and washed three times with distilled water, followed by anhydrous MgSO 4 After drying for 12 hours, filtering and removing the solvent, the crude product was washed three times with petroleum ether, and finally the petroleum ether was removed, and the white liquid product was obtained by vacuum drying, whic...
Embodiment 2
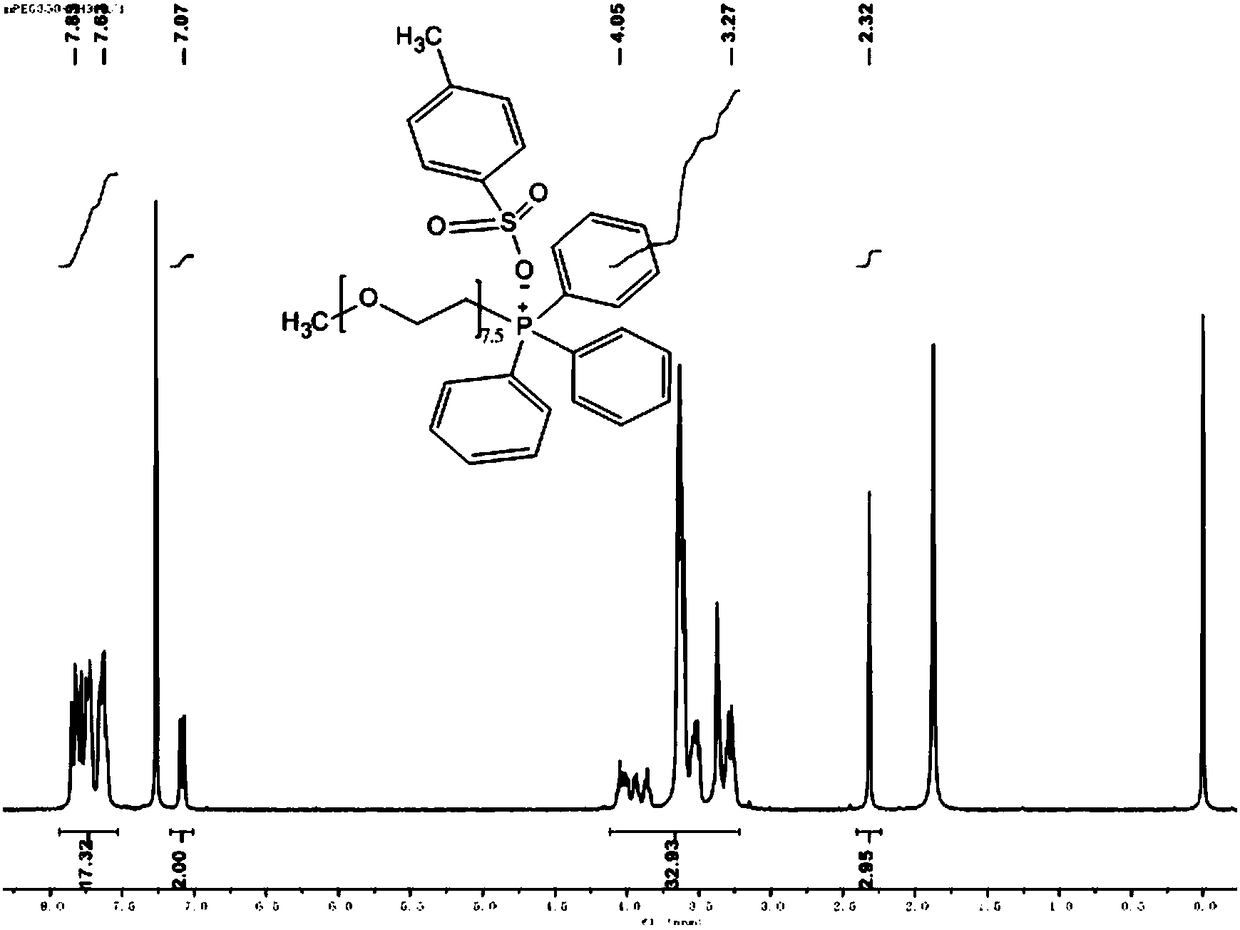
[0099] Embodiment 2: Preparation of polyethylene glycol monomethyl ether (Mw=350) triphenyl-p-toluenesulfonate
[0100] Dissolve 20 mmol of polyethylene glycol monomethyl ether (Mw = 350) in 20 ml of anhydrous CH 2 Cl 2 and added 6.7ml of triethylamine, then slowly added p-toluenesulfonyl chloride TsCl (22mmol of TsCl dissolved in 30ml of anhydrous CH 2 Cl 2 ) solution and drop it off within 1 hour. After removing the ice-water bath and returning to room temperature, stirring was continued for 24 hours. The resulting mixture was washed three times with 1 mol of HCL solution, 30 ml each time, and then the organic layer was washed with anhydrous NaHCO 3 Dry, filter and concentrate. Finally, the crude product was washed three times with petroleum ether, 30 ml each time; the solvent was evaporated and concentrated to obtain a light yellow oily compound, which was polyethylene glycol monomethyl ether (Mw=350) p-toluenesulfonate.
[0101] Dissolve 5mmol of polyethylene glycol ...
Embodiment 3
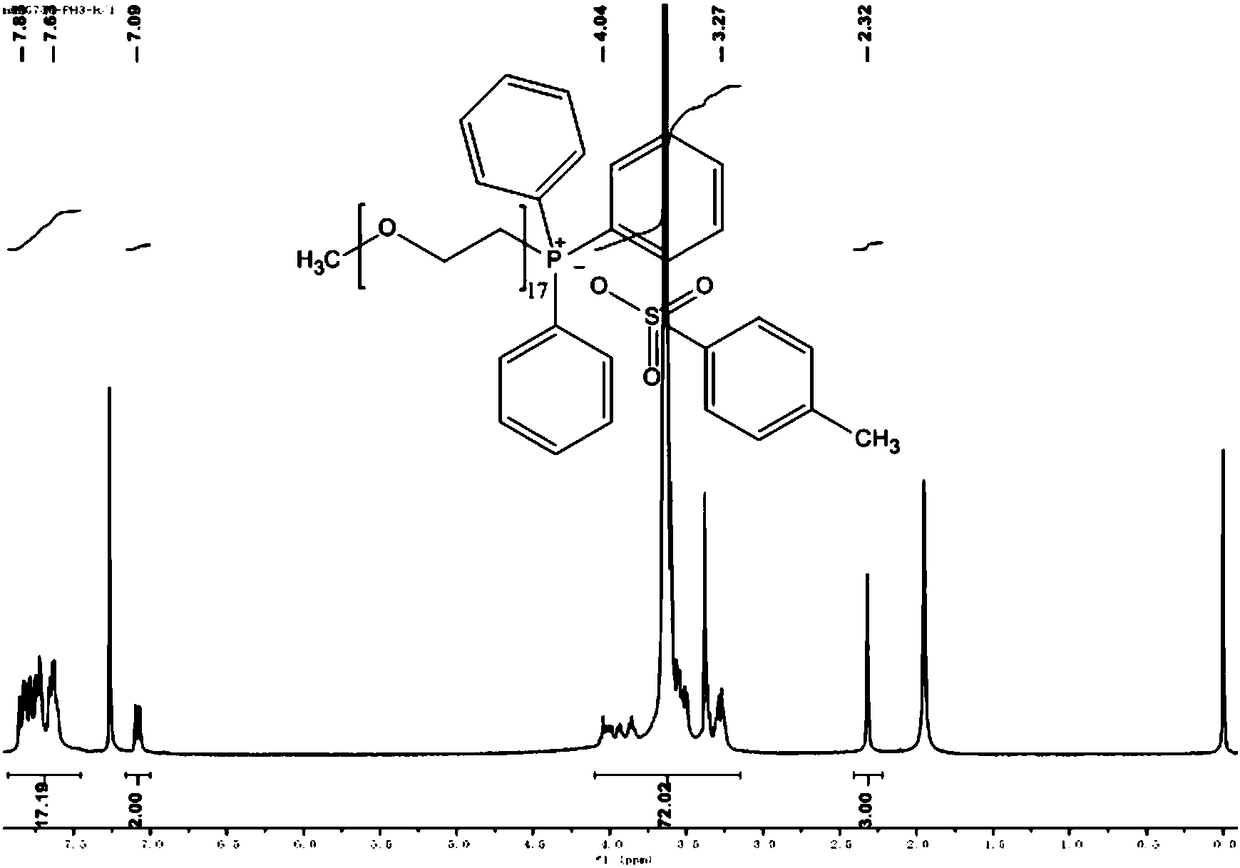
[0102] Embodiment 3: Preparation of polyethylene glycol monomethyl ether (Mw=750) triphenylphosphine p-toluenesulfonate
[0103] Dissolve 20 mmol of polyethylene glycol monomethyl ether (Mw=750) in 20 ml of anhydrous CH 2 Cl 2 and added triethylamine (6.7ml), then slowly added p-toluenesulfonyl chloride TsCl (4.2g, 22mmol TsCl dissolved in 30ml anhydrous CH 2 Cl 2 ) solution and drop it off within 1 hour. After removing the ice-water bath and returning to room temperature, stirring was continued for 24 hours. The resulting mixture was washed three times with 1 mol of HCl solution, 30 ml each time, and then the organic layer was washed with anhydrous NaHCO 3 Dry, filter and concentrate; finally, the crude product is washed three times with petroleum ether, 30ml each time; the solvent is removed by evaporation and concentration to obtain a light yellow oily compound, which is polyethylene glycol monomethyl ether (Mw=750) p-toluenesulfonate .
[0104] Dissolve 5mmol of poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com