Method for determining ginsenosides in rat plasma
A ginsenoside and plasma technology, applied in the field of ginsenoside detection, can solve the problem of high instrument cost, and achieve the effects of high sensitivity, high accuracy and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
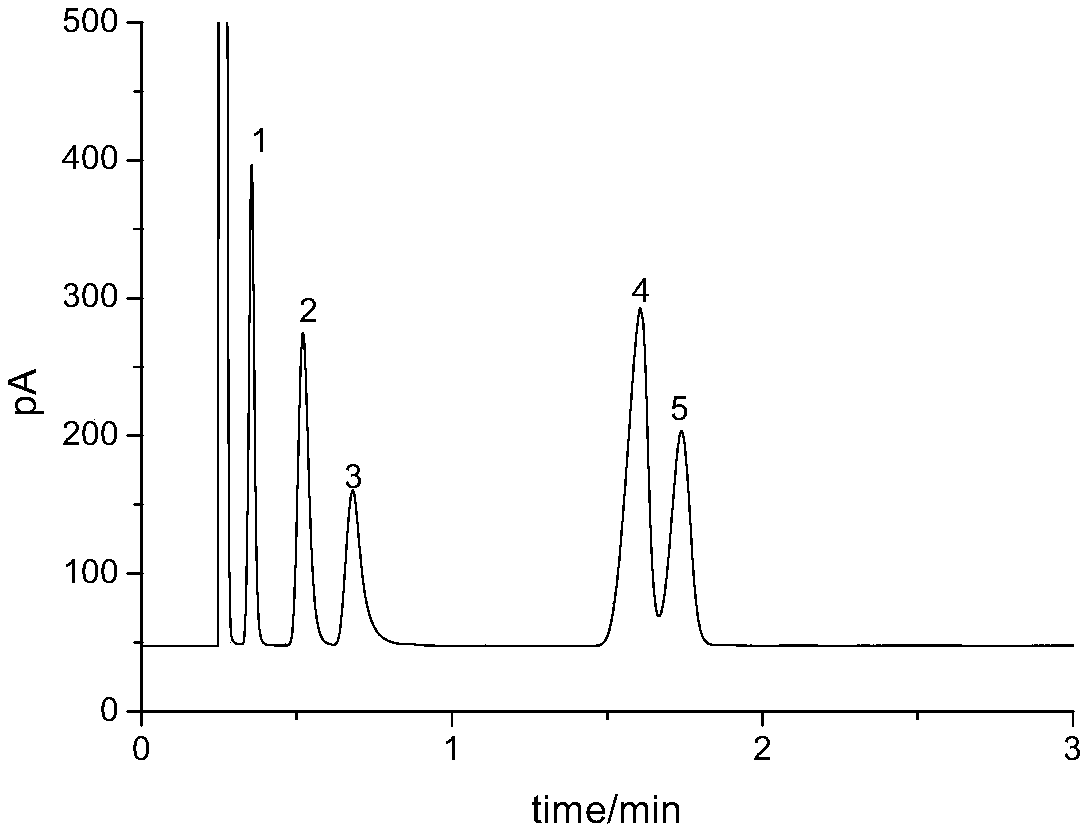
[0026] Take 100 μL of rat plasma, put it in a 2 mL centrifuge tube, add 200 μL methanol, vortex for 1 min, and centrifuge. Take the supernatant, put it into another clean 2mL centrifuge tube, add 500μL of dichloromethane, vortex for 1min, and centrifuge. The above two centrifugation conditions are 1000g, 3min. The Oasis HLB column was activated sequentially with methanol, water, and dichloromethane, and the extracted supernatant was added, eluted with 3 mL of methanol, and the eluate was collected. The eluent was taken, blown with nitrogen gas to near dryness, and the residue was redissolved in methanol.
[0027] The chromatographic conditions are as follows: DB-35MS, 30m×0.25mm, 0.25μm; heating program: the initial temperature is 60°C, rising to 160°C at a rate of 50°C / min, and then rising to 220°C at a rate of 20°C; Temperature 250°C; carrier gas: nitrogen, purity ≥99.999%; flow rate 1.2mL / min; injection volume 1μL; splitless injection.
[0028] The detection conditions a...
Embodiment 2
[0031] standard curve drawing
[0032] Add different volumes of ginsenoside reference solution in the centrifuge tube, after drying in a water bath, add blank plasma to form plasma samples containing different concentrations of ginsenoside, analyze using the pretreatment-analysis method of Example 1, and obtain a chromatogram Figure, record the peak area, take the peak area as the ordinate, and the concentration as the abscissa, draw the standard curve. The regression curves of ginsenosides Ra1, Rb1, Rh1, Re and Ro are as follows: y=0.057x+0.027, y=0.007x+0.215, y=0.098x-0.125, y=0.105x-0.024, y=0.0127x+ 0.114.
[0033] Method recovery
[0034] In order to verify the recovery rate of the method, the sample recovery experiment was carried out at three levels of high, medium and low respectively. The added material was then extracted and quantified. Three repetitions were performed for each of the three levels of high, middle and low. The results showed that the recoveries ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com