Conjugated polymer containing trifluoromethyl group and its preparation method and application
A technology of conjugated polymer and trifluoromethyl, which is applied in the field of trifluoromethyl-containing conjugated polymer and its preparation, can solve the problems of limiting the open circuit voltage of the device, affecting the efficiency of the device, improving the efficiency of the device, and achieving the improvement of photoelectric conversion efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Compound BDTP-m-CF 3 Synthesis
[0055] The reaction scheme is as follows. In the formula, R represents a 2-butyloctane group. The specific reaction steps and reaction conditions are as follows:
[0056]
[0057] (1) Synthesis of compound 2: under argon protection, 4-bromo-2-(trifluoromethyl)phenol (compound 1, 10.85g, 45mmol) and K 2 CO 3 (12.42 g, 90 mmol) was added to 150 mL of DMF. After stirring for 5 minutes, 5-(bromomethyl)undecane (13.46 g, 54 mmol) was added, followed by reaction at 90° C. for 24 hours. The cooled mixture was filtered through a Buchner funnel to remove solids and collect the organic liquid. The organic liquid was extracted three times with ether. The combined organic phases were washed with anhydrous magnesium sulfate (MgSO 4 )dry. After removing the organic solvent, the crude product was purified by a silica gel column and eluted with petroleum ether. Compound 2 shown in the figure was obtained as a pale yellow oil (16.95 g, 92%). ...
Embodiment 2
[0063] Example 2 Polymer PBZ-m-CF 3 Synthesis.
[0064] The chemical reaction scheme of the present embodiment is as follows, and in the formula, R represents 2-butyloctane group, and concrete reaction steps and reaction conditions are as follows:
[0065]
[0066] In a 50 mL double necked round bottom flask, compound BDTP-m-CF 3 (0.3 mmol, 352 mg) and compound FBTZ (0.3 mmol, 210 mg) were dissolved in 10 mL of toluene. After purging with argon for 20 minutes, 18 mg of Pd(PPh 3 ) 4 This was added to the flask as a catalyst, and the reaction mixture was purged with argon for an additional 30 minutes. The reaction mixture was stirred and heated to reflux under an atmosphere of argon for 15 hours. When the reaction mixture was cooled to room temperature, 100 mL of methanol was added to precipitate the polymer, which was collected by filtration, and finally Soxhlet extraction was performed with methanol, n-hexane and chloroform. Polymer recovery from chloroform fractions by...
Embodiment 3
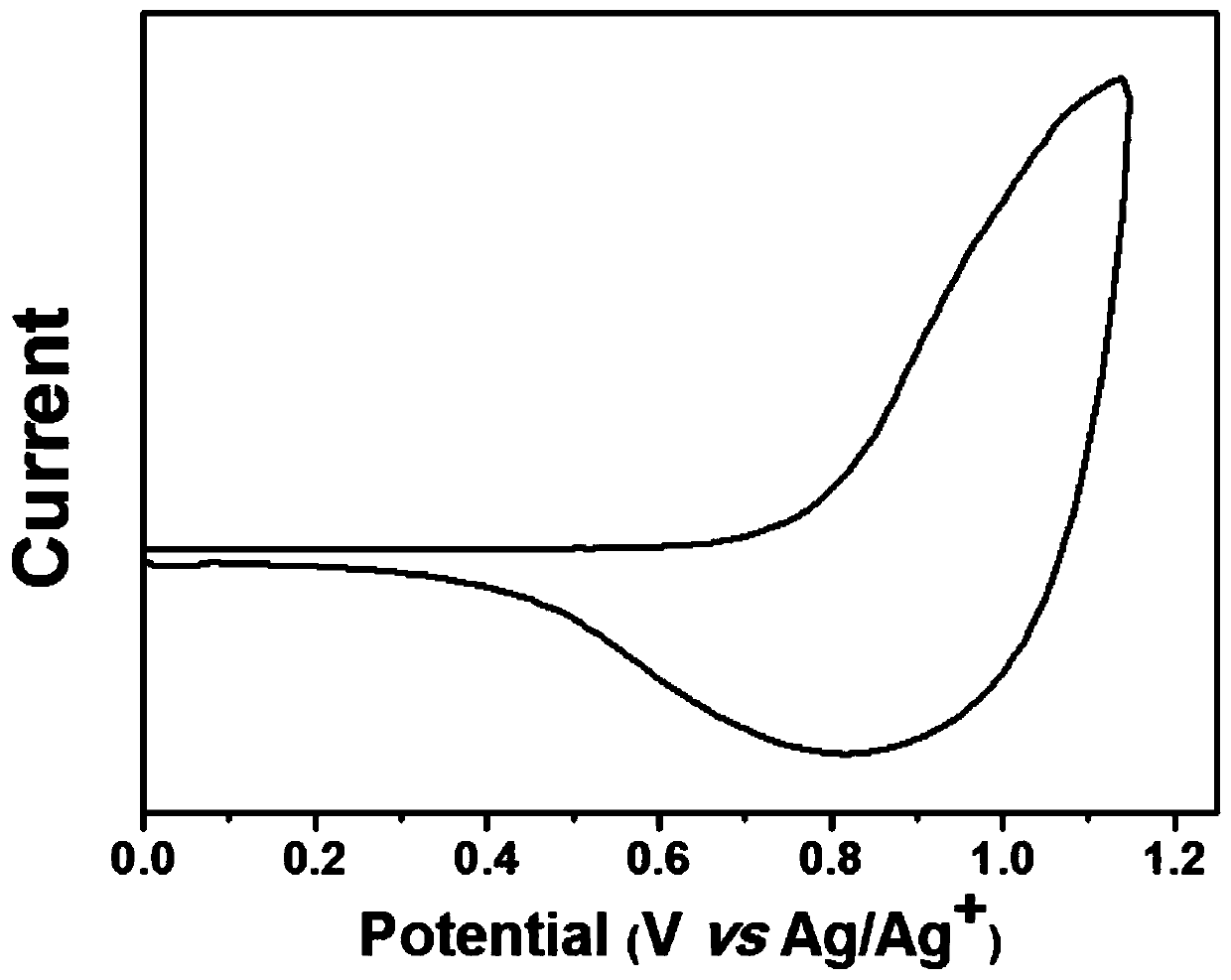
[0074] The polymer PBZ-m-CF prepared by embodiment 2 3 (1.0mg) was dissolved in 1mL of chloroform, and then the solution was added dropwise onto a working electrode, such as a platinum sheet; 0.1mol / L Bu 4 NPF 6 Acetonitrile solution was used as electrolyte; platinum wire was used as counter electrode; silver wire was used as reference electrode. Measured in this system using electrochemical cyclic voltammetry, the polymer PBZ-m-CF 3 The cyclic voltammetry data are shown in image 3 middle. Have image 3 The results can be calculated, the polymer PBZ-m-CF 3 The HOMO energy level of is -5.49eV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com