Tetranuclear dysprosium clusters based on 2-formyl-8-hydroxyquinoline trihydroxyaminomethane Schiff base and its synthesis method and application
A technology for condensing trihydroxyaminomethane with hydroxyquinoline and a synthesis method, which is applied in the field of magnetic materials, and achieves the effects of simple synthesis method, good repeatability, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: The compound shown in formula (I) is the synthesis of 2-formyl-8-hydroxyquinoline trihydroxyaminomethane Schiff base ligand
[0031]
[0032] The specific synthesis method is as follows: Weigh 0.0173g (0.1mmol) of 2-formyl-8-hydroxyquinoline and dissolve it in 5mL of methanol, and dissolve 0.0121g (0.1mmol) of trishydroxymethylaminomethane in 5mL of acetonitrile. The methanol solution of 8-hydroxyquinoline was slowly added to the acetonitrile solution of trishydroxymethylaminomethane. At this time, the mixed solution was red, and it was put into a magnet and stirred for 30 minutes. The solution was light yellow, and a solution containing ligand was obtained. .
Embodiment 2
[0033] Embodiment 2: the compound shown in formula (I) is the synthesis of 2-formyl-8-hydroxyquinoline trihydroxyaminomethane Schiff base ligand
[0034] Repeat Example 1, the difference is that the amount of methanol is changed to 2mL, the amount of acetonitrile is changed to 4mL, and the reaction time is changed to 20min.
Embodiment 3
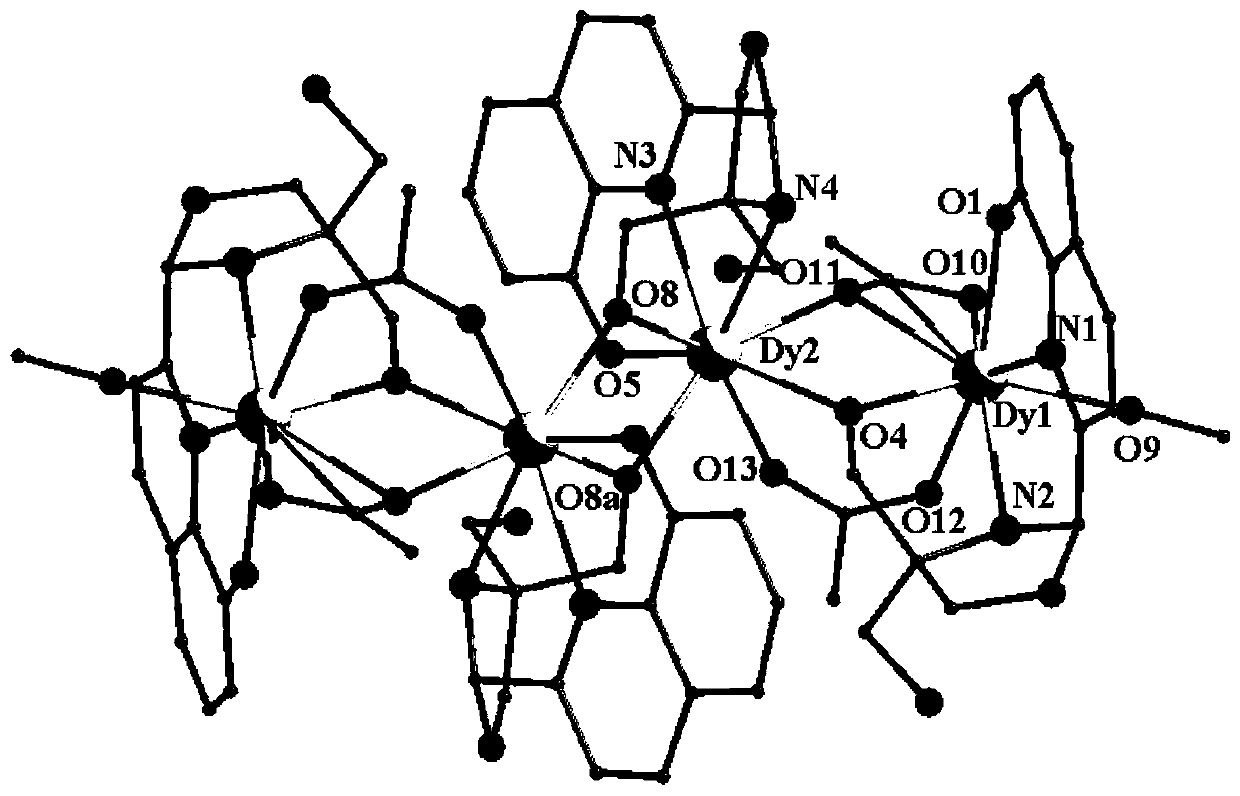
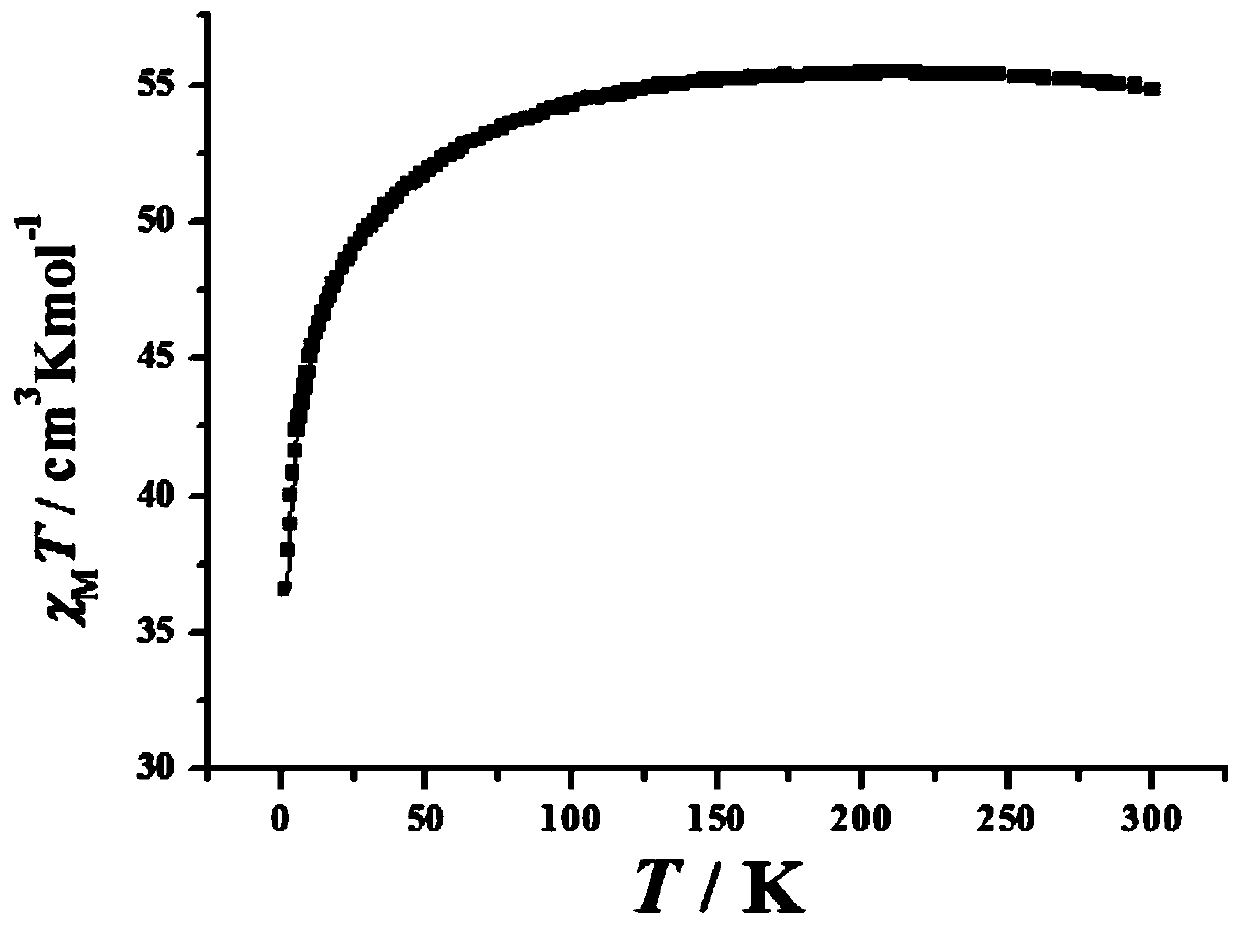
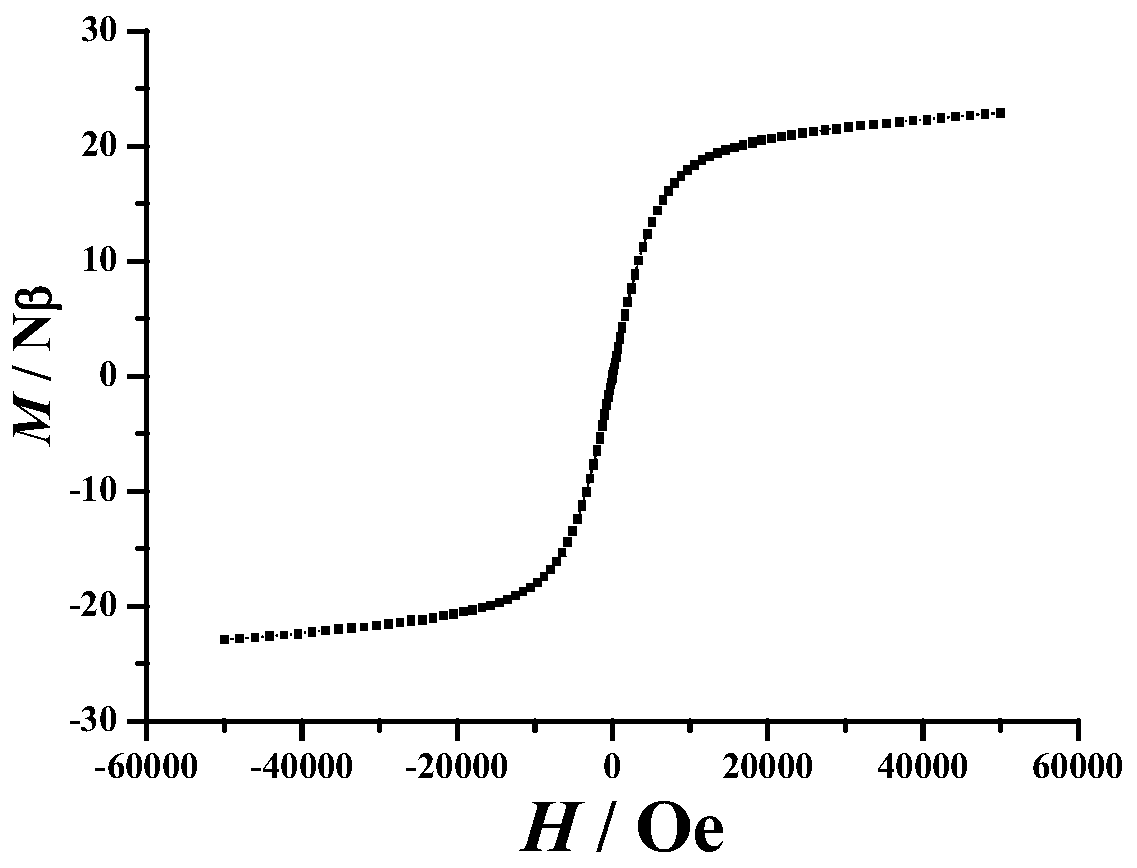
[0035] Embodiment 3: tetranuclear dysprosium cluster [Dy 4 (C 14 h 14 N 2 o 4 ) 4 (CH 3 COO) 4 (CH 3 Oh) 2 ]·2H 2 Synthesis of O
[0036] Weigh Dy(CH 3 COO) 3 ·6H 2 O (0.2mmol, 91.2mg) was placed in a glass bottle with a cover, and the ligand solution prepared in Example 1 was sucked with a disposable rubber tip dropper (1mL ligand solution contained methanol 0.5mL, acetonitrile 0.5mL, Ligand (0.1mmol) was added to the glass bottle, and then 0.25mL of methanol and 1.75mL of acetonitrile were added to it, so that the volume ratio of methanol and acetonitrile in the mixed solvent was 1:3, and then 45μL of triethylamine was added to it after dissolving , shake gently until the precipitate disappears (the pH of the solution at this time is 7.3), then wrap the mouth of the bottle tightly with aluminum foil, cover the bottle cap, place the glass bottle at 60°C for 24 hours, take it out, and wrap it with cotton wool After the glass bottle was cooled to room temperature,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com