Synthesizing method of 2-butyl-1-octanol
A synthesis method, butyl technology, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve problems such as expensive catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Such as figure 1 Shown is the reaction equation of aldol condensation reaction, and concrete process is as follows: Hexanal (13.9g) adds the aqueous solution of barium hydroxide (2.2g Ba(OH) 2 .8H 2 (0, 25ml of water), heating to reflux for 1 hour, liquid separation, and vacuum distillation to collect 70-80°C (310Pa) fractions to obtain 2-butyl-2-octenal (20.3g).
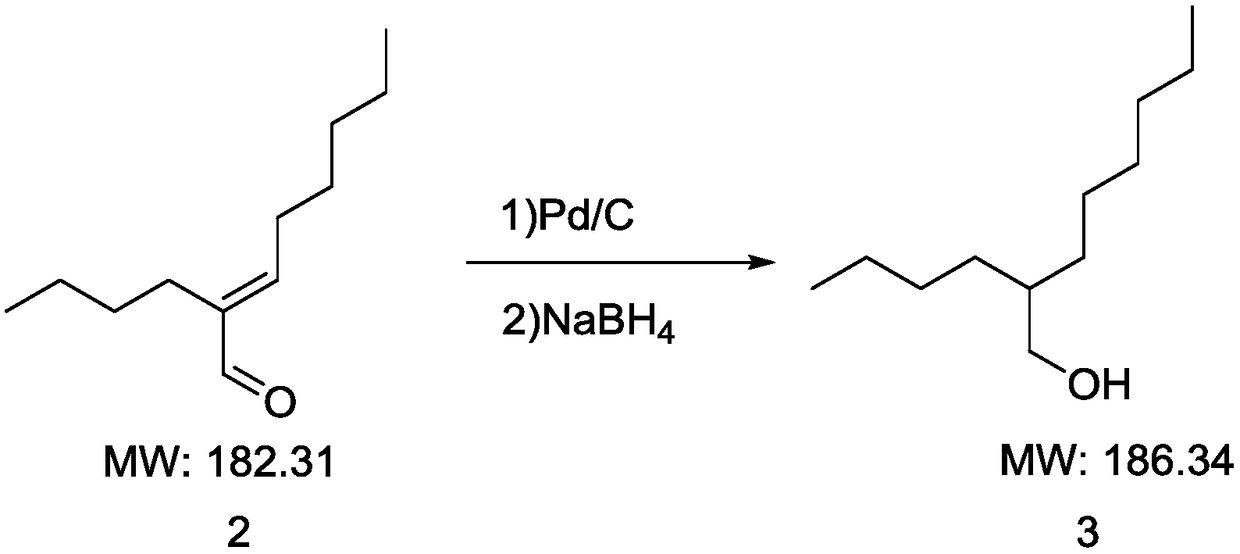
[0022] Such as figure 2 Shown is the reaction equation of primary reduction reaction and secondary reduction reaction of the present invention, concrete process is as follows: the above-mentioned 2-butyl-2-octenal that obtains is dissolved in methanol (160ml), adds palladium carbon (2.031g, 5 %Pd / C), replace the air with vacuum, pressurize (1.5MPa), heat the temperature to 100°C, the raw material is converted into a mixture of aldehyde and alcohol, and this mixture of aldehyde and alcohol is added with NaBH 4 (3.0g) reduction, react at room temperature (25°C) for 1 hour, heat up to 50°C, add water (100ml)...
Embodiment 2
[0024] Hexanal (123ml, 100.1g) was placed in a 1000ml three-necked flask, and an aqueous solution of barium hydroxide (8.9g Ba(OH) 2 .8H 2 (2, 100ml water), dripped in about 20 minutes, heated to reflux for 1 hour, and separated liquids, and the organic layer obtained was collected by distillation under reduced pressure at 70-80°C (310Pa) cuts to obtain 2-butyl-2-octenal ( 163.9g).
[0025] The 2-butyl-2-octenal obtained above was dissolved in methanol (320ml), palladium carbon (8.15g, 5% Pd / C) was added, the air was replaced by vacuum, pressurized (1MPa), and the temperature was heated to 50°C. Converted to a mixture of aldehydes and alcohols, this mixture of aldehydes and alcohols is added with NaBH 4 (30.7g) was reduced, heated to 50°C, added water (200ml) after 1 hour, stirred for 15 minutes, concentrated methanol under reduced pressure, added saturated brine (400ml), extracted twice with ethyl acetate, and combined the ethyl acetate layers , spin-dried under reduced pr...
Embodiment 3
[0027] Hexanal (3.8L, 3.1kg) was placed in a 20L reaction flask, and an aqueous solution of barium hydroxide (170.2g Ba(OH) 2 .8H 2 O, 2.5L water), about 1 hour drop, heated to reflux for 1 hour, liquid separation, the upper layer vacuum distillation collected 70-80 ° C fraction (310Pa), to obtain 2-butyl-2-octenal (5.0kg) .
[0028] The above-mentioned 2-butyl-2-octenal was dissolved in methanol (8.0L), and palladium carbon (24.9g, 5% Pd / C) was added, the air was replaced by vacuum, pressurized (1MPa), and the temperature was heated to 50°C. Converted to a mixture of aldehydes and alcohols, this mixture of aldehydes and alcohols is added with NaBH 4 (93.6g) reduction, no obvious bubbles, heated to 50 ° C, added water (600ml) after 1 hour, stirred for 15 minutes, concentrated methanol under reduced pressure, added brine (1000ml), extracted twice with ethyl acetate, combined ethyl acetate The ester layer was spin-dried under reduced pressure, distilled by an oil pump under r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com