Lithium borohydride compound fast ion conductor and preparation method thereof
An ion conductor and lithium borohydride technology, which is applied in the field of lithium borohydride composite fast ion conductor and its preparation, and can solve problems such as hidden dangers of battery safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In the isolation of air (H 2 O2 4 The mixture of -NaX is calculated according to the set molar ratio (1~4:1), and put into a stainless steel ball tank equipped with stainless steel grinding balls; the mechanical ball milling method of the planetary wheel ball mill is used to obtain high-purity ( 99.9999%) under the protection of inert gas, LiBH was obtained respectively 4 -NaCl or LiBH 4 - NaI complex particles. The total mass of the sample in the spherical tank is 1g, the volume of the ball milling tank is 100ml, the weight ratio of the balls to the sample is 40:1, the ball milling time is 20-40 hours, and the revolution speed is set at 400-450rpm.
Embodiment 2
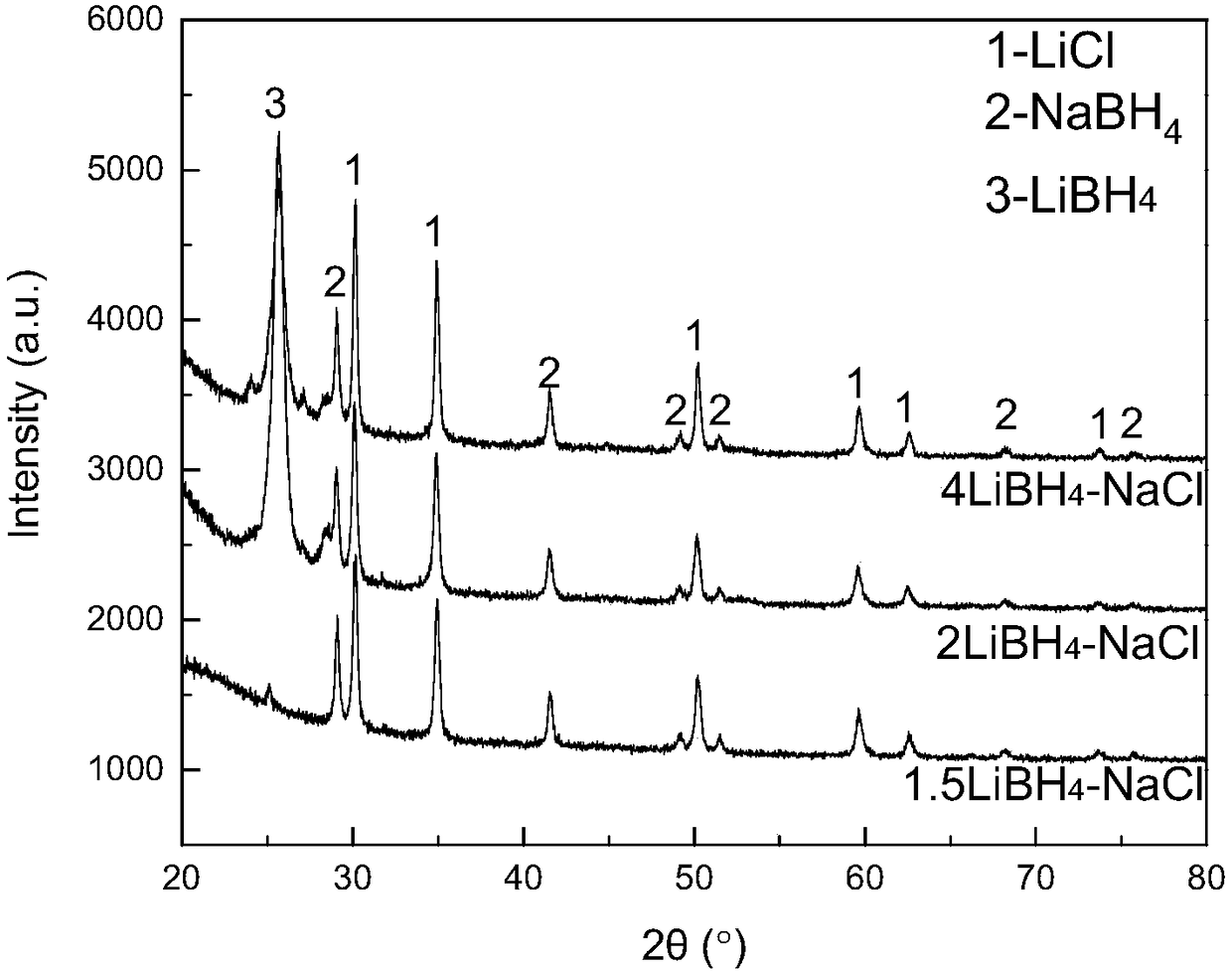
[0031] Take out the LiBH prepared according to the method of Example 1 4 -NaX (X refers to Cl - ,I - Ion) complex particles for X-ray diffraction (XRD) experiments, the sample cell is covered by a specific polymer film, and it is sealed with the glass slide with vacuum grease to prevent the effect of water and oxygen in the air on the sample. The target material of the X-ray source used is a Cu target, the tube voltage is 40kV, and the tube current is 40mA. The resulting XRD spectrum is as figure 1 , 2 shown.
Embodiment 3
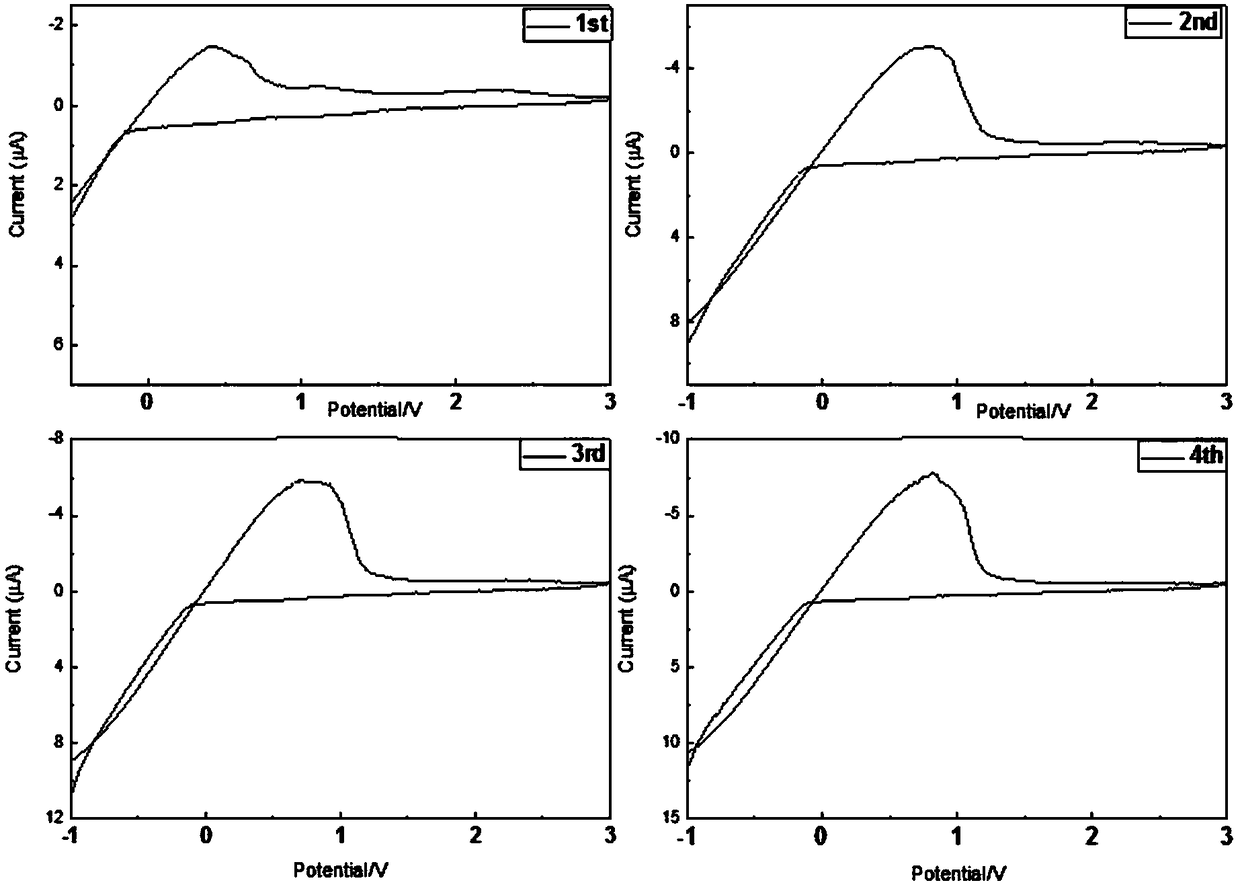
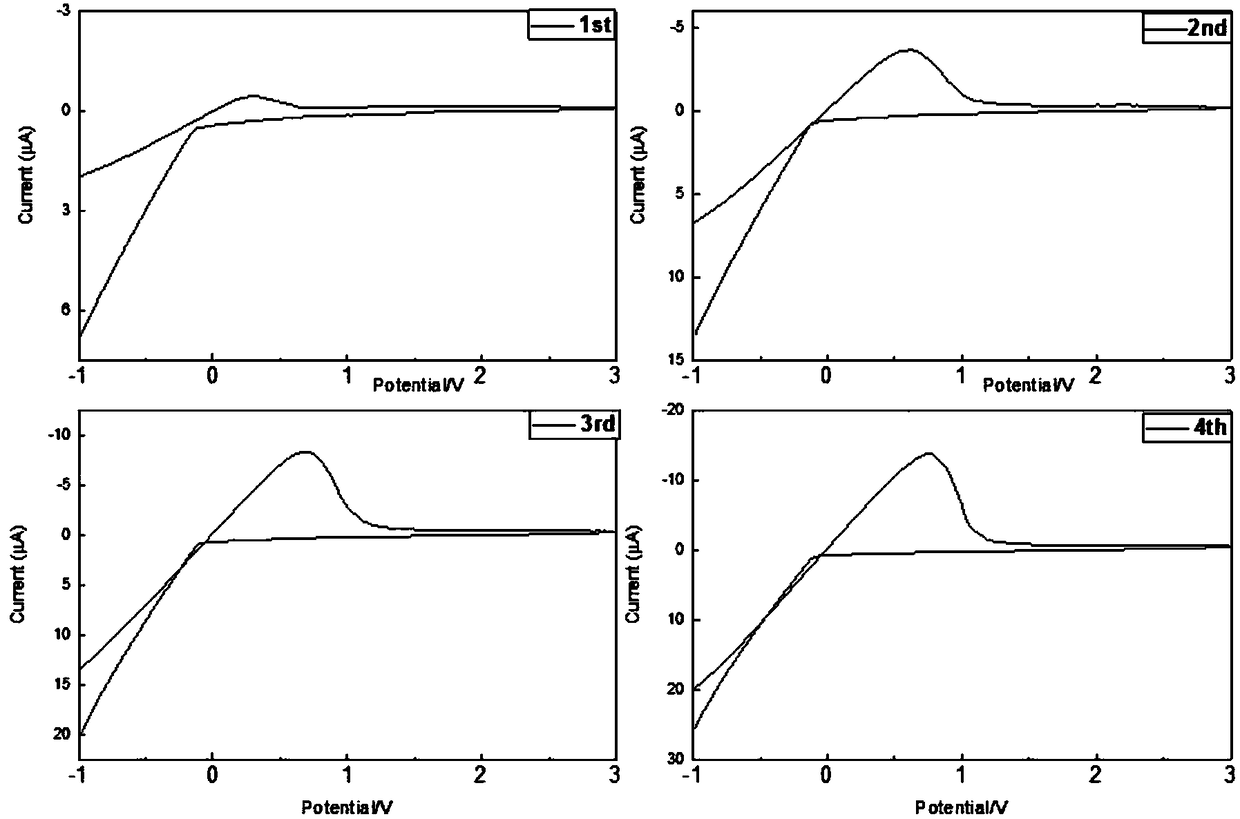
[0033] A series of LiBH obtained in embodiment 1 4 -NaX (X refers to Cl - ,I - The ion) ball milled sample was pressed into a disc with a diameter of 10 mm and a thickness of about 1 mm with a pressure of 20 MPa. Two lithium foils were placed on both sides of the sample disc as electrodes. 1 ton / cm 2The pressure of the lithium foil and the sample sheet are pressed tightly. All preparations were performed under high purity argon (99.9999%). The frequency range of the AC impedance test is from 1MHz to 100Hz. The sample was heated at a rate of 2°C / min, and impedance spectra were collected every 5°C. The temperature rose from room temperature to 120°C. Obtain the ion transport resistance and conductivity through the obtained AC impedance Nyquist spectrum, and make the change curve of conductivity with temperature as shown in image 3 , 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com