Magnetic micro particle immunofluorescence kit for quantitatively assaying classical swine fever virus antibody
A technology of fluorescence immunity and swine fever virus, which is applied in the field of animal disease detection, can solve the problems that it is not suitable for widespread use by grass-roots veterinary departments, cannot realize on-site detection of samples, increases detection time and difficulty, and achieves long fluorescence decay time and overcomes difficulties. Effects of stability and shortening of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 preparation method:
[0035] A detection kit for swine fever virus antibody, comprising: reagent strips, negative control serum and positive control serum.
[0036] The reagent strip is mainly coated with immunomagnetic beads of classical swine fever virus antigen E2 protein, europium-labeled sheep anti-pig antibody, sample diluent, washing solution, and enhancing solution;
[0037] (1) Preparation method of classical swine fever virus antigen E2 protein:
[0038] ① Amplification of CSFV E2 protein
[0039] A pair of primers were designed according to the gene sequence of the attenuated CSFV strain published on GenBank (GenBank accession number is AF531433) for amplifying the E2 gene.
[0040] Primers P1: 5'-CGCGGATCCCGGCTAGCCTGC-3'; P2: 5'-CCGCTCGAGTTATAGTACCTGTTCT-3'.
[0041] ②Construction of recombinant plasmid pEGFP-E2
[0042] The amplified classical swine fever virus E2 gene was connected to the eukaryotic expression vector pEGFP-C1 vector by clo...
Embodiment 2
[0065] Embodiment 2 This kit usage method:
[0066] ① Add sample
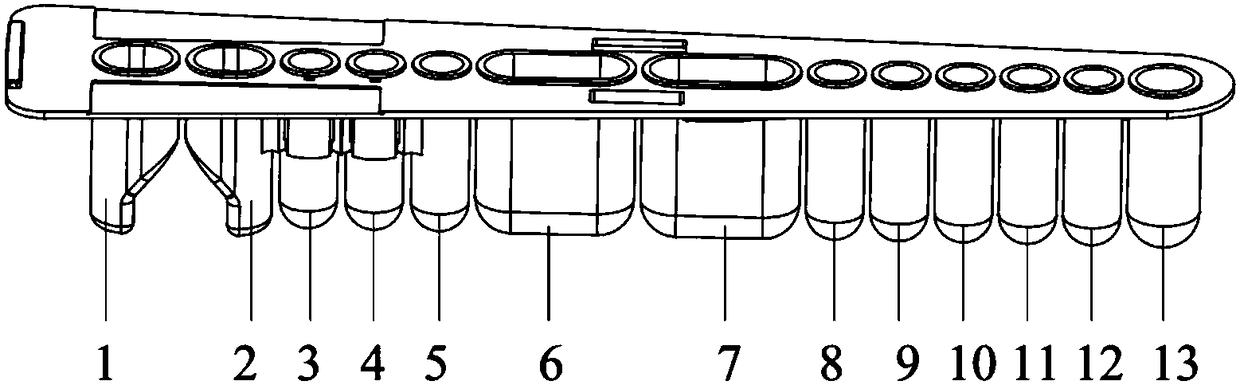
[0067] Put the sample to be tested into the loading system of the automatic fluorescence analyzer, insert the reagent strip into the reagent strip slot, and the instrument will automatically identify the product information of the sealing film. Add 20 μL of the sample to the 8th well, dilute 32 times, then take 50 μL of the diluted sample and add it to the 1st well of the reagent strip, and then add 100 μL of the fluorescent marker.
[0068] ② Incubation
[0069] After adding samples, shake and incubate at 37°C for 15 minutes.
[0070] ③ washing
[0071] After the incubation is completed, the instrument automatically washes the wells 5 times, each washing solution is 100 μL / well.
[0072] ④Add enhancement solution
[0073] After washing, 150 μL / well of enhancement solution was added and incubated at 37°C for 3 minutes.
[0074] ⑤ Detection
[0075] The reagent strip is pushed into the dark room, and the ...
Embodiment 3
[0079] Embodiment 3 The specificity experiment of this kit
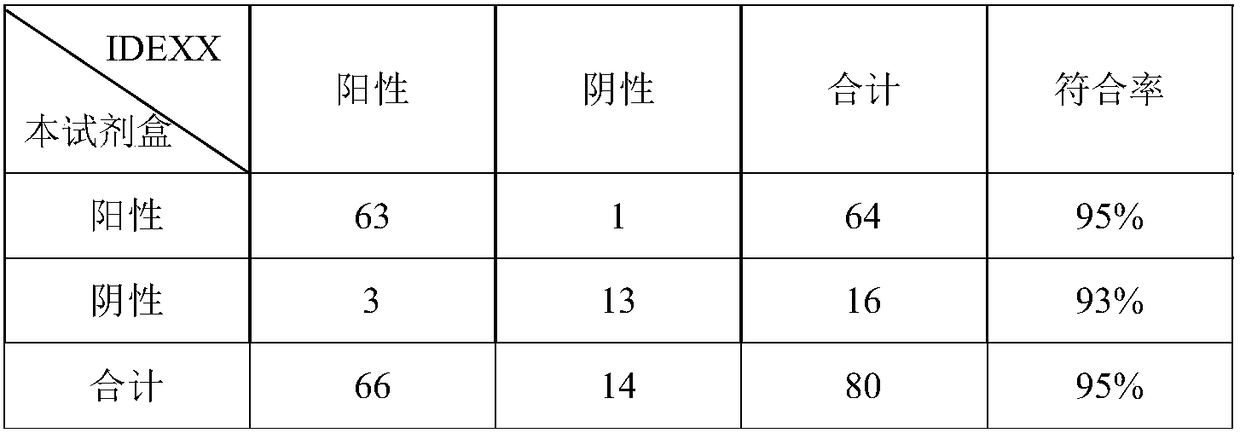
[0080] Magnetic Particle Fluorescent Immunoassay Kit for Quantitative Detection of Antibody to CSFV in Specific Experiments to Detect CSFV (CSFV), Bovine Viral Diarrhea Virus (BVDV), Sheep Border Virus (BDV), Porcine Pseudorabies (PRV) and Pig Blue Ear (PRRSV), porcine parvovirus (PPV) and other standard positive serum and CSF negative serum, except CSFV standard serum is 95840, the fluorescence value of the rest of the serum is less than 20022, in line with the criteria for negative serum, indicating that this method is specific Good, and there is no cross-reaction with BVDV and BDV positive sera, the test results are shown in Table 1.
[0081] Table 1 Specificity experiments
[0082] virus type
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com