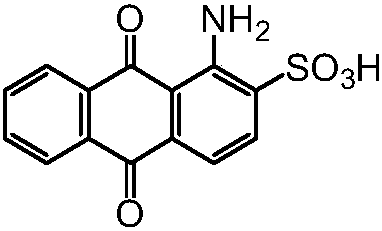
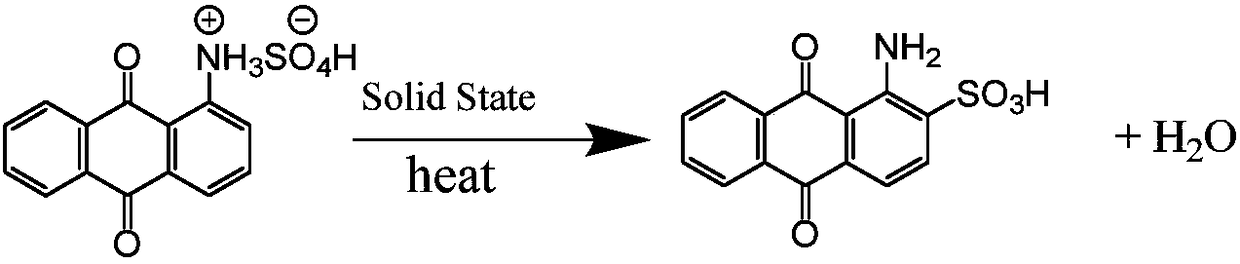
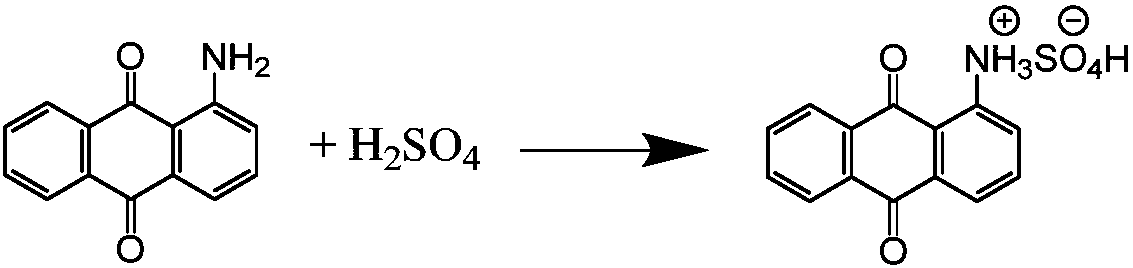Process for synthesizing 1-amino-anthraquinone-2-sulfonic acid through solid-phase method
A technology of aminoanthraquinone and solid-phase method, which is applied to the preparation of sulfonic acid, chemical instruments and methods, and the preparation of organic compounds. To achieve the effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In the solid phase reactor of 10L, add the ceramic ball that the diameter of 3kg is 10cm, add the content of 2.28kg then and be 98% 1-aminoanthraquinone (10mol) and the mass fraction of 1.2kg be 98% sulfuric acid (12mol ), the reactor was rotated for 3 hours to fully mix 1-aminoanthraquinone and sulfuric acid to form a salt, and the temperature was raised to 180°C. After reacting for 15 hours, the content of 1-aminoanthraquinone-2-sulfonic acid in the product was measured by liquid chromatography to be 97.3%, and the content of 1-aminoanthraquinone was 2.7% (area normalization method).
[0034] Attachment: Further synthesis of bromic acid:
[0035] Use 30L of water to wash the product in the solid-phase reactor several times, put it in a 200L bromination pot, add ice water to adjust the volume to 90L, the temperature to 0-5°C, adjust the pH value to 2, and add bromine dropwise at the same time element and NaClO aqueous solution, and keep the temperature at 0-5°C, adjus...
Embodiment 2
[0037] In a 20L reactor, add 10kg of dichloroethane, 2.28kg of 98% 1-aminoanthraquinone (10mol) and 1.01kg of 98% mass fraction of sulfuric acid (10.1mol), fully stir to make 1 -Aminoanthraquinone and sulfuric acid are fully mixed to form a salt. At the same time, the temperature is raised to distill dichloroethane, and then the temperature is raised to 200°C, and the vacuum is evacuated to an absolute vacuum of 10mmHg to take out the water vapor generated by the reaction. Keep the reaction for 8h. The content of 1-aminoanthraquinone-2-sulfonic acid in the product measured by liquid chromatography is 99.2%, and the content of 1-aminoanthraquinone is 0.8% (area normalization method).
[0038] Attachment: Further synthesis of bromic acid:
[0039] Using the same method as in Example 1, 3.95kg of bromic acid product was obtained, content (calculated as bromic acid sodium salt, MW404): 96.5%, liquid chromatography purity: 100%, from 1-aminoanthraquinone to bromic acid The overall...
Embodiment 3
[0041] In a 20L reactor, add 10kg of chlorobenzene, 2.28kg of 98% 1-aminoanthraquinone (10mol) and 1.05kg of 98% sulfuric acid (10.5mol) in mass fraction, fully stir to make 1-aminoanthraquinone Anthraquinone and sulfuric acid are fully mixed to form a salt. At the same time, the temperature is raised to distill chlorobenzene, and then the temperature is raised to 180°C, and the vacuum is evacuated to an absolute vacuum of 10mmHg to take out the water vapor generated by the reaction. Keep the reaction for 15h, and use liquid chromatography The measured content of 1-aminoanthraquinone-2-sulfonic acid in the product is 99.4%, and the content of 1-aminoanthraquinone is 0.6% (area normalization method).
[0042] Attachment: Further synthesis of bromic acid:
[0043]Add 6kg of 98% sulfuric acid to the above reaction kettle, heat up to 80°C, add 0.6kg of bromine dropwise in 8 hours under reflux, react at 80°C for 2h, cool down to room temperature, add 12kg of water dropwise, filter ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com