A kind of dextran polymer, polymer micelle and drug carrier system
A technology of polymer glue and carrier system, which is applied in the direction of drug combination, cardiovascular system diseases, antipyretics, etc., can solve the problems of insufficient stability, limit the application of polymeric micelles, and poor targeting, and achieve good hydrogen peroxide Responsiveness, good responsiveness, damage reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
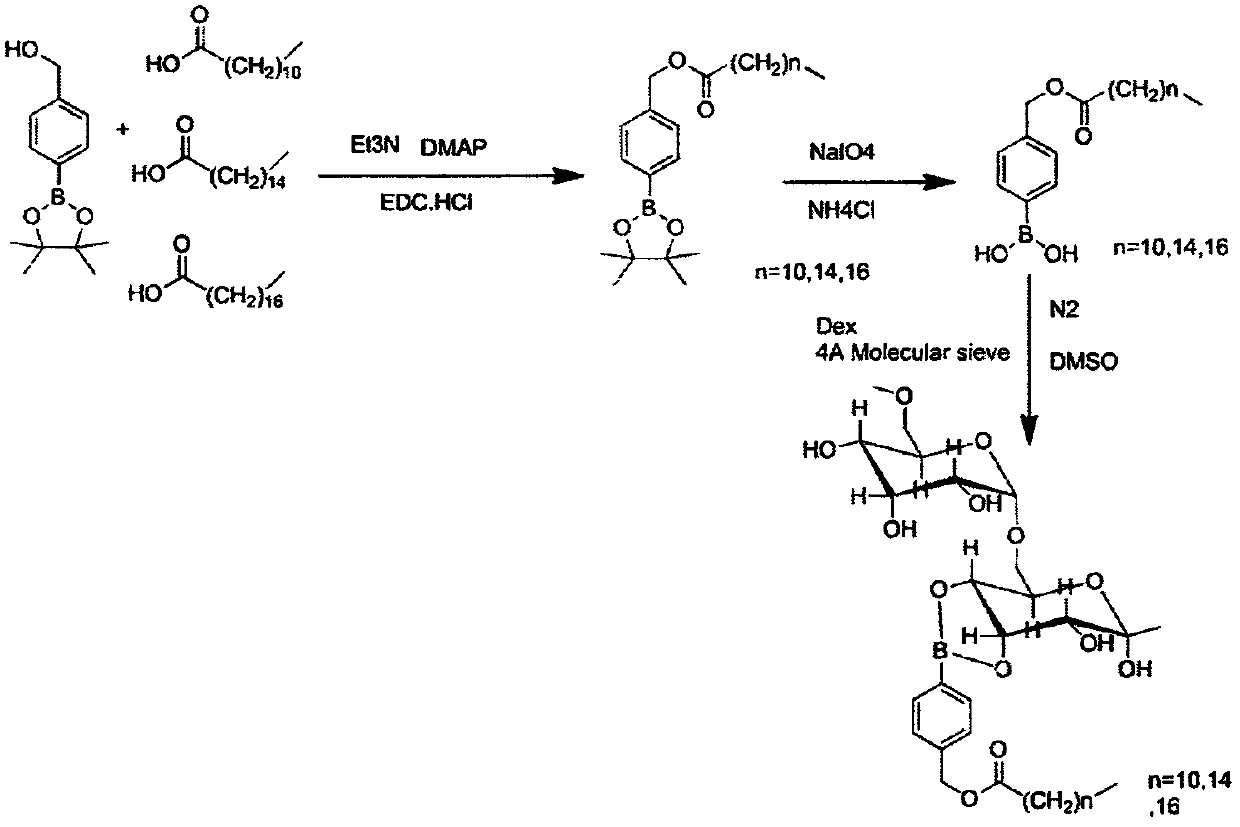
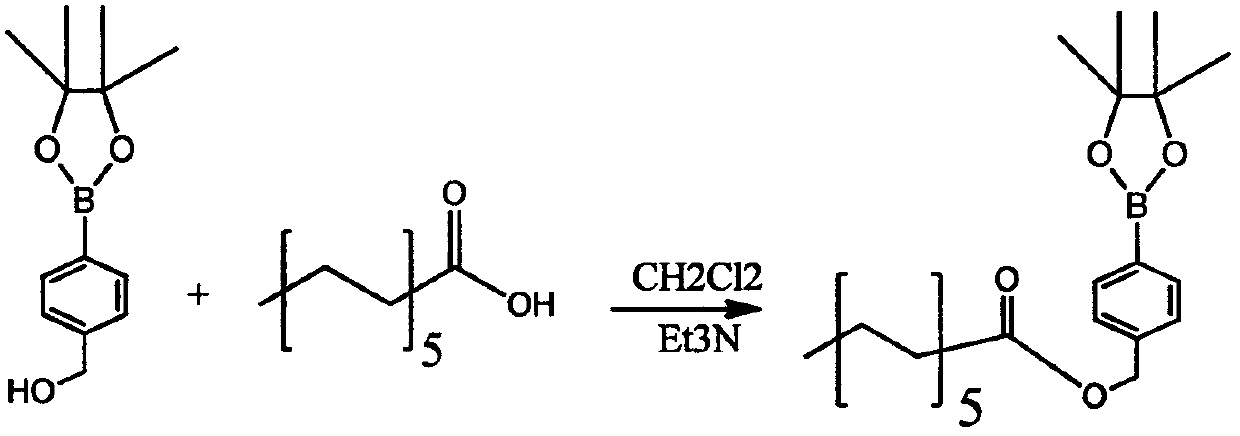
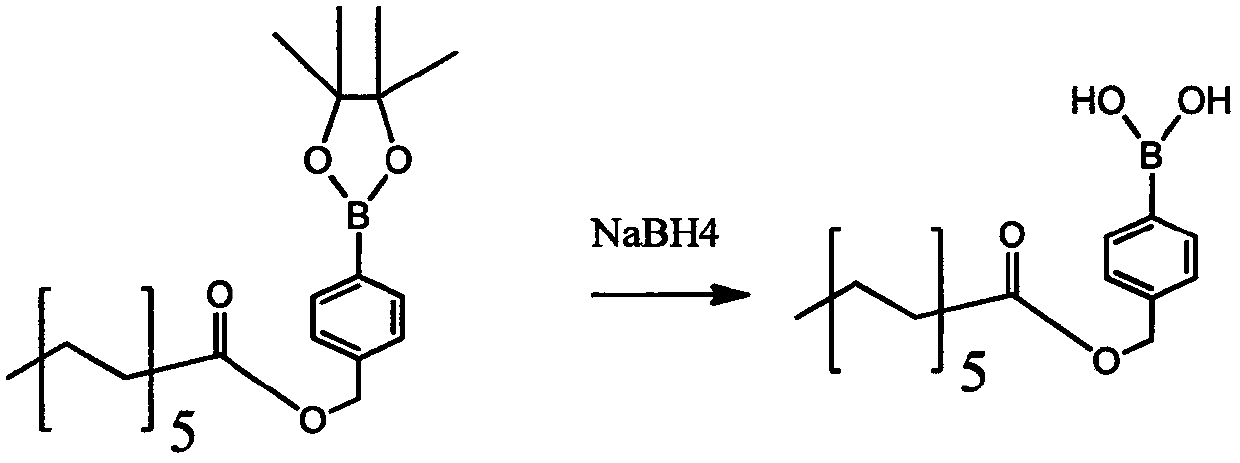
[0059] A kind of preparation method of dextran polymer and micelle with hydrogen peroxide responsiveness, its synthetic route is as follows figure 1 As shown, the specific steps are as follows:
[0060] 1) First react boroester with dodecanoic acid, under the protection of nitrogen, put boroester raw material A (1eq) and dodecanoic acid (1.5eq) into a 25mL round bottom bottle, and dissolve with DCM. Weigh DMAP (0.2eq) EDCI (1.5eq) and Et3N (0.5eq) in order and dissolve them in DCM respectively, then inject into the system with a syringe. The reaction solution was reacted at room temperature for 18 hours, followed by TLC spot plate. After the reaction is complete, add water to quench. The aqueous layer was extracted 4 times with DCM, the organic phases were combined, and the 4 Dry, filter with suction, and spin-dry to obtain the crude product, which is then purified by column chromatography. After passing through the mobile phase of PE:EA=50:1 to remove impurities, use PE:EA...
Embodiment 2
[0066] Use fluorescent probe technology to measure the critical micelle concentration (CMC) of micelle described in embodiment 1, concrete steps are as follows:
[0067] 1) Preparation of methanol solution of fluorescent probe pyrene
[0068] Dissolve 0.0051 g of pyrene in 25 ml of methanol to obtain a pyrene solution with a concentration of 1.01×10-3 mol / L, and then dilute it with methanol to obtain a pyrene solution of 1.616×10-6 mol / L.
[0069] 2) Preparation of DA-B-DEX aqueous solutions with different concentrations
[0070] The preparation concentration was 1×10 -4 , 1×10 -3 , 1×10 -2 , 1×10 -1 , 1, 10, 100, 1000mg / ml DA-B-DEX solution.
[0071] 3) Preparation of pyrene-containing micelles
[0072] Take 150 μl of the prepared pyrene solution, put it into several 10ml volumetric flasks, and evaporate the methanol to dryness. Then, add 10ml of DA-B-DEX solution of each concentration into the 10ml volumetric flask containing a trace amount of pyrene. Sonicate in a w...
Embodiment 3
[0076] A method for preparing a polymer micelle drug carrier with hydrogen peroxide responsiveness, the steps are as follows:
[0077] 1) DA-B-DEX (100mg) was dissolved in DMSO (10ml), and added dropwise into PBS buffer (100ml, pH7.4) under stirring, and kept stirring for 30min. Spontaneous formation of micelles was confirmed by observation of a clear opalescent solution.
[0078] 2) Simultaneously prepare DA-B-DEX micelles that physically entrap doxorubicin, DA-B-DEX (91.5mg) and DOX (8.5mg) are dissolved in 10mL dimethyl sulfoxide (DMSO) together, under stirring Add dropwise to 100mL PBS buffer, and keep stirring for 30min. Dialyze through a dialysis bag (molecular weight cut-off = 3.5KD) for 24 hours to remove organic solvents and free drugs, and finally freeze-dry and store.
[0079] 3) The particle size distribution is measured by using a Malvern laser particle size analyzer (Zetasized Nano, ZS, Malvern, United Kingdom). The morphology and size of micelles were evaluat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com