Preparation method of antitumor targeting drug nilotinib arylamine intermediate
A hydrotalcite and bimetallic technology, applied in the field of new drug research and development, can solve problems such as long reaction time, step yield needs to be improved, cumbersome post-processing, etc., and achieve good versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Prepare the hydrotalcite-like catalyst of coated Fe / Pd bimetal as follows:
[0045] 1) Preparation of Pd-containing hydrotalcite-like: Add 50mmol palladium acetate, 400mmol Ni(NO 3 ) 2 ·6H 2 O, 400mmol Mg(NO 3 ) 2 ·6H 2 O and 300mmol Mn(NO 3 ) 2 4H 2 O, stir and dissolve to obtain a mixed solution; heat up to 40-45°C, then dropwise add 2mol / L potassium hydroxide aqueous solution to maintain the pH of the mixed solution between 10.2-10.8, keep stirring for 18-24h; filter, wash with water, and Dry in air at 70-80°C to constant weight; heat up to 100-120°C and dry for 6-8h, then heat up to 400-600°C at a heating rate of 20°C / h for 2-3h to obtain Pd-containing Hydrotalcite-like;
[0046] 2) Preparation of iron oxide nanoparticles: 20 mmol of iron triacetylacetonate and 100 mmol of octadecenoic acid were added to 200 ml of diphenyl ether, then 30 ml of reagent grade hydrazine monohydrate (98% wt) was added dropwise, and the temperature was raised to React under nit...
Embodiment 2
[0063] Nitro reduction reaction process optimization: use the catalyst prepared by sequence 3 in Example 1 as the catalyst for the reduction of 5-trifluoromethyl-3-(4-methylimidazol-1-yl) nitrobenzene, for reaction solvent, catalyst Consumption, reducing agent consumption, reaction temperature have been optimized, and reaction conditions are as follows:
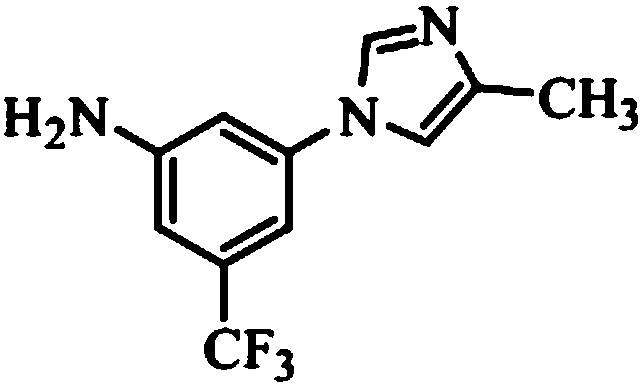
[0064] 1) Substrate 5-trifluoromethyl-3-(4-methylimidazol-1-yl) nitrobenzene (2.71g, 10mmol), reducing agent borane ammonia complex (1-4eq of substrate ), a catalyst (0.5-20%wt of the weight of the substrate) is placed in a 50ml solvent and reacted at 10-70°C;
[0065] 2) Take the reaction solution every 1h, and detect the remaining amount of the substrate 5-trifluoromethyl-3-(4-methylimidazol-1-yl)nitrobenzene by HPLC, when the concentration in the reaction solution no longer decreases Stop the reaction, the results are shown in Table 2;
[0066] Table 2 Optimization results of different reaction conditions
[0067]
...
Embodiment 3
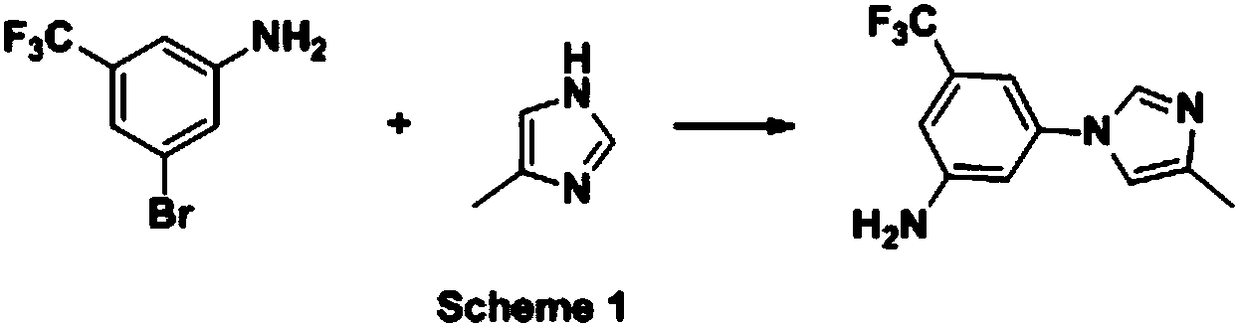
[0070] On the basis of embodiment 2 optimization, select MeCN / H 2 O (V:V=10:1) is the solvent, the amount of the catalyst is 10%wt, the amount of the reducing agent borane ammonia complex is 3eq, the conversion rate of raw materials reaches 99.8%, and the selectivity reaches about 99.3%, but the product is about About 0.4% of the isomer is transferred from the isomer 2-methyl-1H-imidazole of the initial raw material 4-methyl-1H-imidazole, and the reaction scheme is as follows:
[0071]
[0072] Although the reaction conversion rate and selectivity have reached more than 99%, due to the presence of about 0.45% isomers in the raw materials, the catalyst is filtered out from the reaction solution after reduction, and the product is concentrated to obtain a crude product, which is then recrystallized with ethanol. , The product after crystallization contains about 0.42% isomers, which is basically the same as before reduction, and basically has no removal effect on isomers.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com