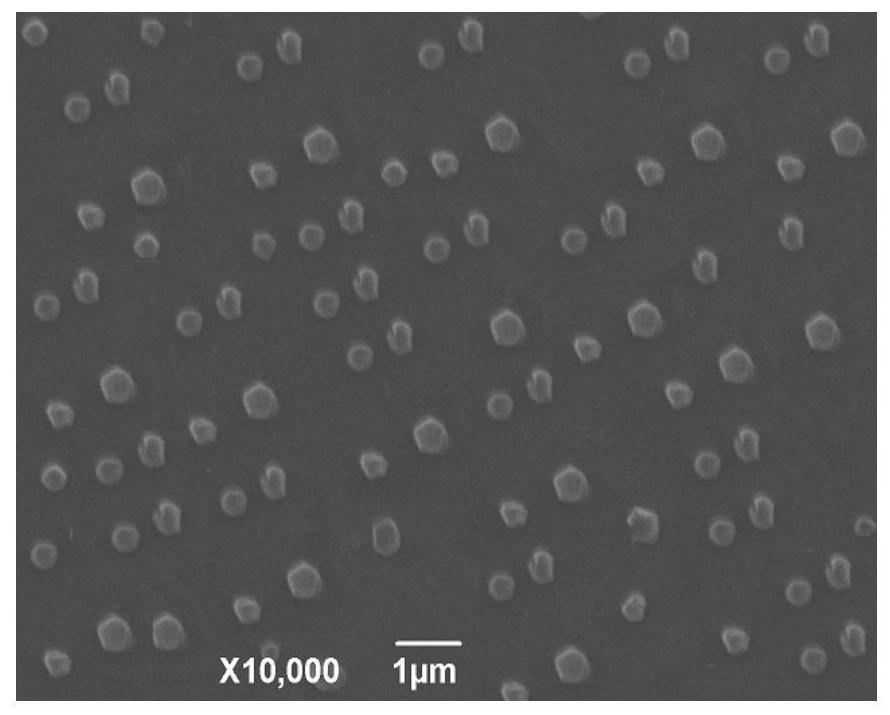
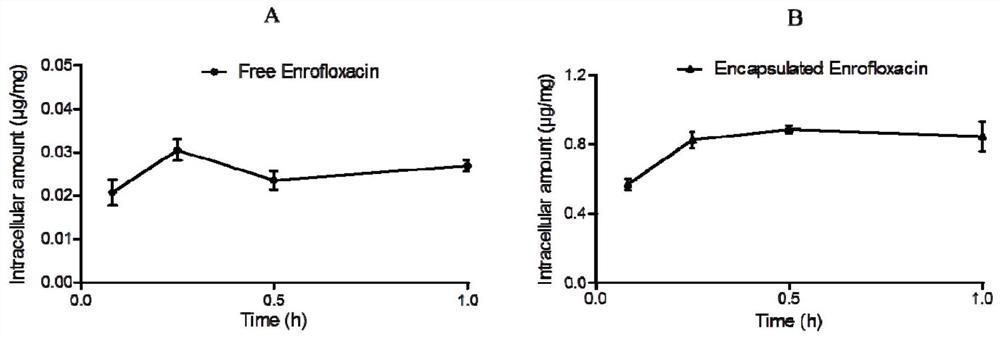
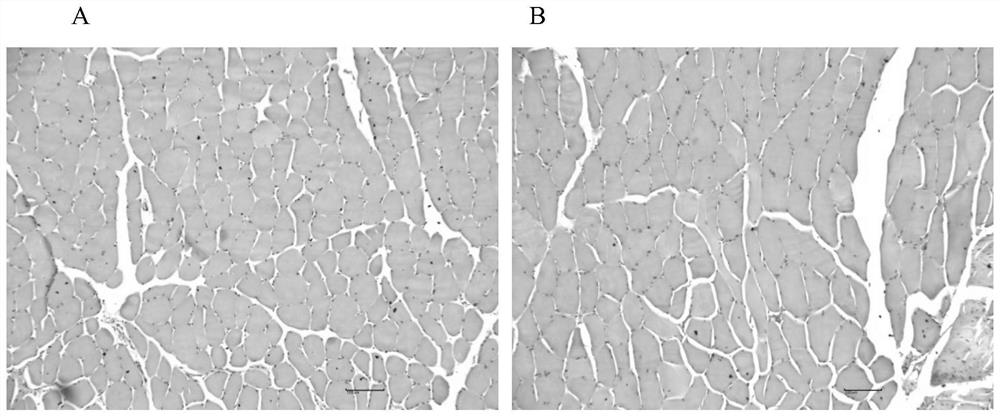
Veterinary enrofloxacin solid lipid nanosuspension and preparation method thereof
A technology of solid lipid nanometer and enrofloxacin, which is applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc. Widespread clinical application and other issues, to achieve the effect of improving bioavailability, improving absorption, and reducing clinical doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The formula of veterinary enrofloxacin solid lipid nanosuspension is shown in Table 1, and its preparation method is as follows:
[0060] (1) Weigh 12g of behenic acid and place it in a test tube heated by an oil bath at 120°C, add 2g of enrofloxacin bulk drug to it after heating and melting, and form a transparent enrofloxacin solution under constant stirring;
[0061] (2) Weigh 0.2g sodium lauryl sulfate and sodium bisulfite respectively and add them to 30mL of sterilized water to form a solution;
[0062] (3) Put the solution prepared in step (2) into the enrofloxacin solution prepared in step (1) after being heated in an oil bath at 120° C. at a stirring speed of 3000 to 5000 r / min to form an oil-in-water colostrum;
[0063] (4) Dispersing the colostrum prepared in step (3) with a disperser for 2 to 5 minutes to form an oil-in-water emulsion;
[0064] (5) Quickly pour the emulsion prepared in step (4) into 30 mL of sterilized water at 4°C to obtain the enrofloxaci...
Embodiment 2
[0070] The formula of veterinary enrofloxacin solid lipid nanosuspension is shown in table 2, and its preparation method is as follows:
[0071] (1) Weigh 12g of behenyl alcohol and place it in a test tube heated by an oil bath at 120°C, add 2g of enrofloxacin bulk drug to it after heating and melting, and form a transparent enrofloxacin solution under constant stirring;
[0072] (2) Weigh 0.5g poloxamer 188, 0.2g sodium formaldehyde sulfoxylate and 3.5g dimethyl dioctadecyl ammonium chloride respectively and add them to 30mL of sterilized water to form a solution;
[0073] (3) Put the solution prepared in step (2) into the enrofloxacin solution prepared in step (1) after being heated in an oil bath at 120° C. at a stirring speed of 3000 to 5000 r / min to form an oil-in-water colostrum;
[0074] (4) Dispersing the colostrum prepared in step (3) with a disperser for 2 to 5 minutes to form an oil-in-water emulsion;
[0075] (5) Quickly pour the emulsion prepared in step (4) int...
Embodiment 3
[0081] The formula of veterinary enrofloxacin solid lipid nanosuspension is shown in table 3, and its preparation method is as follows:
[0082] (1) Weigh 20g of behenyl alcohol and 15g of behenic acid respectively and place them in a test tube heated in an oil bath at 120°C. After heating and melting, add 6g of enrofloxacin raw material to it, and form transparent enrofloxacin under constant stirring. star solution;
[0083] (2) Weigh 2g polyvinylpyrrolidone K respectively 15 Add 1g of sodium formaldehyde sulfoxylate to 40mL of sterilized water to form a solution;
[0084] (3) Put the solution prepared in step (2) into the enrofloxacin solution prepared in step (1) after being heated in an oil bath at 120° C. at a stirring speed of 3000 to 5000 r / min to form an oil-in-water colostrum;
[0085] (4) Dispersing the colostrum prepared in step (3) with a disperser for 2 to 5 minutes to form an oil-in-water emulsion;
[0086] (5) Quickly pour the emulsion prepared in step (4) i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com