Method of preparing star polyacrylamide by single-electron-transfer living free radical polymerization
A single-electron transfer and polyacrylamide technology, which is applied in the field of single-electron transfer active radical polymerization to prepare star-shaped polyacrylamide, can solve the problems of poor controllability and achieve environmental friendliness, controllable molecular weight, and solvation strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
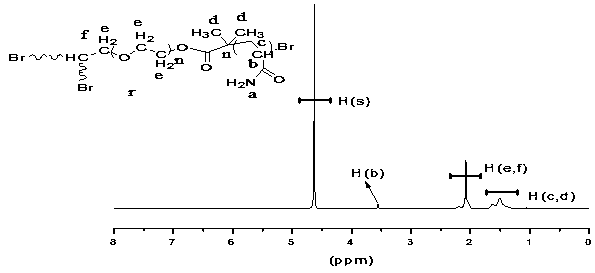
[0032] At room temperature, with the magneton labeled as In the reaction flask, add deionized H 2 O (2ml) and ligand (Me 6 -TREN, 5.3µl, 0.02mmol) was injected with nitrogen for 10 minutes, then CuBr (0.0043g, 0.03mmol) was added, and nitrogen was continued to disproportionate CuBr for 0.5h under anaerobic conditions. Simultaneously the monomer (AM, 0.3741g, 5.3mmol), the initiator Gly-Br 3 (0.0211g, 0.0111 mmol) and a certain amount of deionized H 2 O A total of 3ml was added and marked as In the reaction flask, and make it mix uniformly, pass nitrogen 10min. After the disproportionation is completed, the reaction bottle The medium solution is injected into the reaction vial with a syringe Continue to pass nitrogen in the medium, and stir on the magnetic stirrer, take it out after a certain period of time, and precipitate the polymer with excess acetone, and then pass through the neutral Al 2 o 3 Column chromatography to remove unreacted Cu 0 Powder and the compl...
Embodiment 2
[0038] The SET-LRP polymerization of AM was carried out with reference to the ratio of Example 1, except that the polymerization reaction was carried out at 0°C.
[0039] The monomer conversion rate was 100% as measured by gravimetric method, and the polymer M was measured by GPC. n GPC =38900, M w / M n =1.19
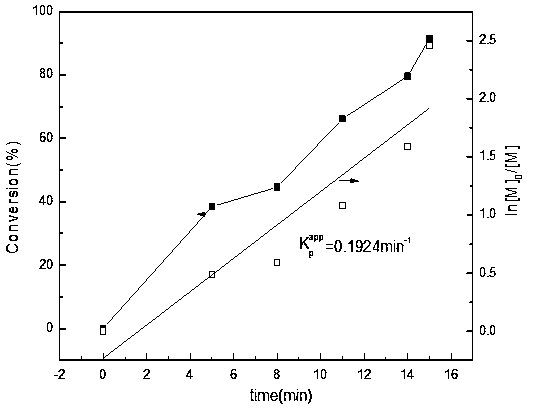
[0040] Figure 4 Be this polyreaction kinetics curve figure, and embodiment one image 3 In comparison, the polymerization temperature is reduced from room temperature (25 °C) to 0 °C, the chain growth rate constant from 0.1924min -1 increased to 0.2601min -1 ; Molecular weight distribution reduces PDI=1.19 (12min conversion rate can reach 100%, M n GPC =38900). This is because the Cu(0) nanoparticles produced by the disproportionation of CuBr at 0°C are smaller than those at room temperature, which is equivalent to increasing the total surface area of Cu(0) and accelerating the polymerization reaction rate. Another reason is that the reaction is carried ou...
Embodiment 3
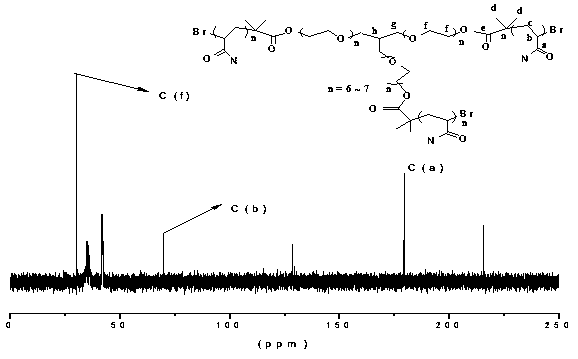
[0043] Add deionized H 2 O (2ml) and ligand (Me 6 -TREN, 10.7µl, 0.04mmol) was injected with nitrogen for 10 minutes, then CuBr (0.0057g, 0.04mmol) was added, and nitrogen was continued to disproportionate CuBr for 0.5h under anaerobic conditions. Simultaneously, the monomer (AM, 0.7463g, 10.5mmol; NVP, 0.0333g, 0.3mmol; 2-acrylamido-2-methylpropanesulfonic acid sodium (NaAMPS), 0.15ml, 0.3mmol) and the initiator Gly -Br 3 (0.0211g, 0.0111 mmol) and a certain amount of deionized H 2 A total of 3ml of O was added to the reaction flask marked II, and mixed evenly, and nitrogen gas was introduced for 10 minutes. After the disproportionation is completed, inject the solution in the reaction bottle II into the reaction bottle I with a syringe to continue to pass nitrogen, and stir on a magnetic stirrer, take it out after a certain period of time, and precipitate the polymer with excess acetone, and pass through neutral Al 2 o 3 Column chromatography to remove unreacted Cu 0 P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com