Docetaxel derivative and its preparation method and application
A technology of docetaxel and derivatives, which is applied in the field of medicine, can solve the problems of non-tumor cell cytotoxicity, achieve the effects of inhibiting proliferation, increasing drug loading and encapsulation efficiency, and reducing non-specific toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of Triphenylphosphine-Docetaxel Derivatives (TD)
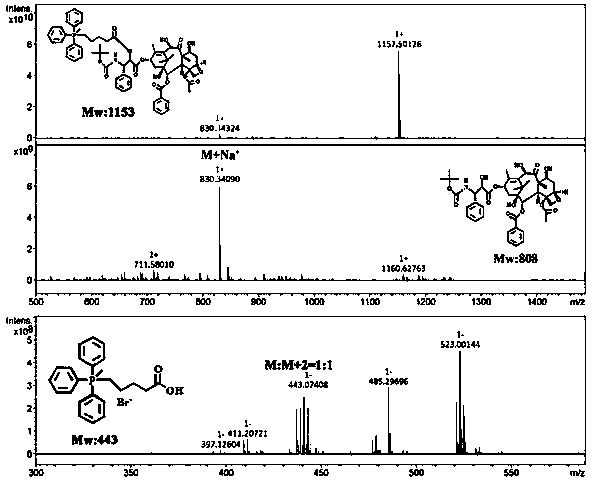
[0041] Dissolve an appropriate amount of docetaxel and 4-carboxybutyltriphenylphosphine bromide in anhydrous DMSO, add appropriate amount of EDC and DMAP, stir at room temperature for 3 days under nitrogen protection, and dialyze the product with distilled water to remove EDC and DMAP , extracted three times with dichloromethane, the organic phase was saturated with sodium sulfate to remove water, and after separation and purification, a white powder was obtained.
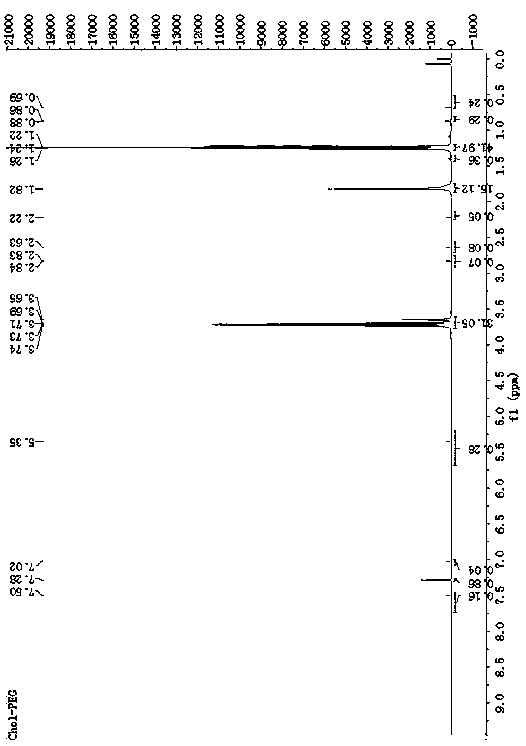
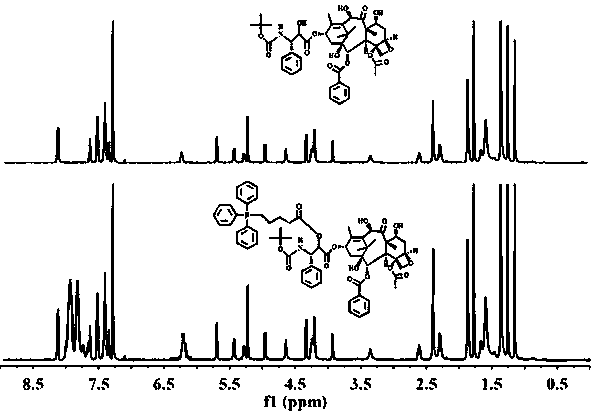
[0042] Determination by NMR 1 H-NMR hydrogen spectrum is determined the structure of TD among the embodiment 1, and the solvent selected is, deuterated DMSO, and the result is as follows figure 1 . 1.15ppm, 1.78ppm, 5.23ppm, 8.13ppm belong to the characteristic peak of docetaxel. 7.7-8.2ppm is the characteristic peak of triphenylphosphine. In the HNMR spectrum of TD, the characteristic peak of carboxylic acid in 4-carboxybutyltriphenylphosphine ...
Embodiment 2
[0045] Synthesis of pH Sensitive Polyethylene Glycol-Cholesterol Derivatives
[0046] Dissolve p-hydroxybenzaldehyde in tetrahydrofuran and add N,N-diisopropylethylamine (DIPEA) to catalyze it. Add a dichloromethane solution of cholesterol formyl chloride under ice-bath conditions and stir at room temperature for reaction. The reaction solution was poured into an appropriate amount of double-distilled water, extracted repeatedly with dichloromethane, the water layer was discarded, and the dichloromethane was distilled off under reduced pressure to obtain an intermediate product A.
[0047] The intermediate product A was dissolved in toluene, phenylenediamine was added, heated in an oil bath at 120°C for reflux reaction, the toluene was distilled off under reduced pressure, acetonitrile was added, solid was precipitated at low temperature, and solid compound B was obtained by suction filtration. The intermediate product C was obtained by separation and purification.
[0048] C...
Embodiment 3
[0051] Preparation of PEGylated liposomes
[0052] Weigh an appropriate amount of TD / DTX, phospholipids, cholesterol, and PEG derivatives into an eggplant-shaped flask, add dichloromethane to dissolve, and place on a rotary evaporator to remove organic solvents to form a uniform film. Add an appropriate amount of redistilled water to hydrate to obtain a suspension, and ultrasonically pass the suspension through the membrane to obtain PEGylated liposomes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com