Application of glabridin liposomes in the preparation of drugs for the treatment of acute (chronic) cardiotoxicity induced by doxorubicin
A glabridin and cardiotoxicity technology, applied in the directions of liposome delivery, drug combination, pharmaceutical formulation, etc., can solve the problems of decreased myocardial contractility, increased myocardial fibrosis, congestive heart failure, etc. The effect of reducing myocardial toxicity and long degradation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] [Example 1] Glabridin liposome preparation and characterization detection
[0025] (1) Glabridin liposome preparation
[0026] 1. Preparation method:
[0027] Weigh cholesterol, lecithin and glabridin and put them in a container, add chloroform-methanol mixed solution, the volume ratio of chloroform to methanol in the chloroform-methanol mixed solution=2:1, rotate at 50-60°C by rotary thin film evaporation method Make a film for 20-50min to make a uniform lipid dry film; the thickness of the lipid dry film is 0.1-0.2mm; dry the residual solvent with nitrogen, then add phosphate buffered saline solution with pH=7 to make the lipid film Fully swollen and hydrated, ultrasonicated for 5 minutes under the condition of ultrasonic power 400W, to obtain final product; in the ultrasonic, the frequency of ultrasonic for 5 seconds and stop for 5 seconds is carried out; the mass ratio of cholesterol to lecithin is cholesterol:lecithin=1: 5. The molar ratio of glabridin to lecithi...
Embodiment 2
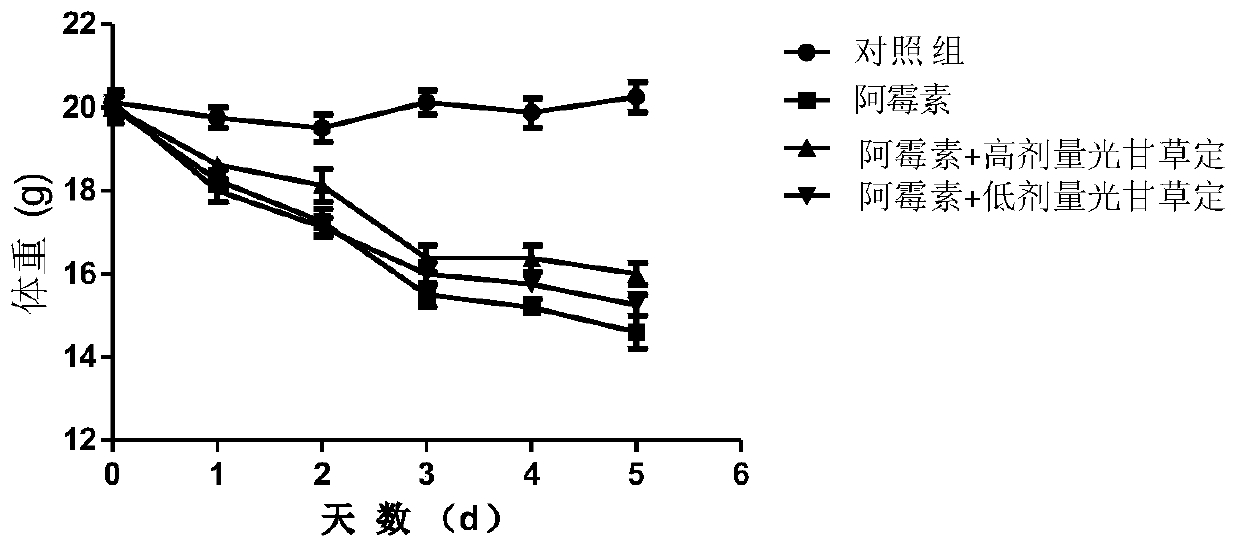
[0071] [Example 2] Research on the effect of glabridin liposome on reducing the acute myocardial toxicity caused by doxorubicin
[0072] (1) Grouping and handling of animals
[0073] 6-8 weeks old C57BL / 6 male mice, 10 in each group, were divided into normal control group, model control group and glabridin liposome treatment group. (1) The normal control group was injected intraperitoneally with the same amount of normal saline every day for 7 consecutive days; (2) The model control group was injected with the same amount of normal saline intraperitoneally every day for 6 consecutive days, and DOX (doxorubicin) 20 mg / kg was injected intraperitoneally on the 7th day; ( 3) The glabridin liposome low-dose experimental group was injected intraperitoneally with 15 mg / kg glabridin liposome every day for 6 consecutive days, and on the seventh day, intraperitoneally injected DOX (doxorubicin) 20 mg / kg; (4) glabridin The liposome high-dose experimental group was injected intraperitone...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com