Preparation method of edaravone injection
A technology for edaravone and injection, which is applied in the field of preparation of edaravone injection, can solve problems such as potential safety hazards, unsuitability for clinical application and the like, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
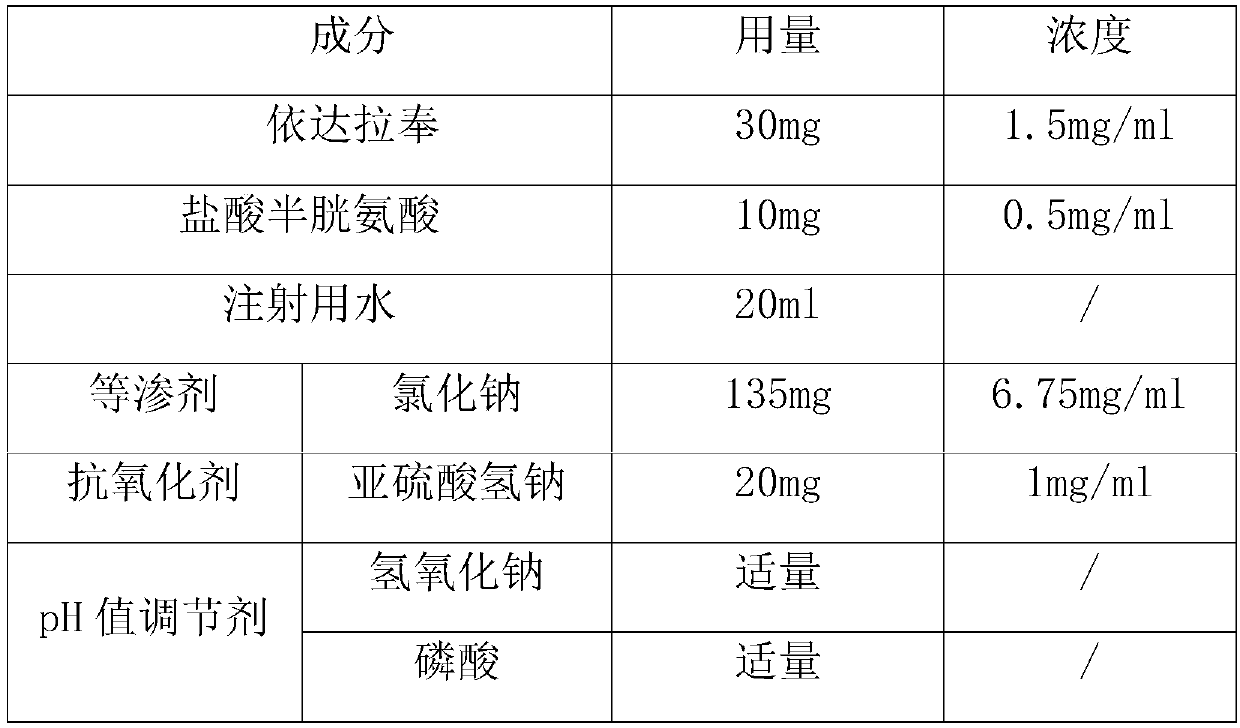
[0044] The preparation method of the embodiment of the present application at least includes the steps of liquid preparation, potting and sterilization, wherein the processes of liquid preparation and potting are all carried out under nitrogen protection. Since the edaravone raw material is easily oxidized, it is carried out under the protection of nitrogen gas throughout the preparation and filling process.
[0045] Further optionally, the nitrogen pressure is not less than 0.02Mpa during the concentrated preparation process, the nitrogen pressure is not less than 0.01Mpa and not greater than 0.02Mpa during the thin preparation process, and the nitrogen pressure is not less than 0.2Mpa during the potting process. In order to ensure the stability of the equipment, the maximum nitrogen pressure should not exceed 0.5Mpa. The present invention has found through research that differential control of the pressure of nitrogen filling in different processes can more effectively improve...
Embodiment 1
[0066] Edaravone injection was prepared according to the original research prescription of Edaravone injection in Table 1 above. The preparation method is:
[0067] 1. Concentrated
[0068] 1.1 Preparation of oxygen-expelling water for injection: put water for injection at 90°C to 100°C in a container, vacuumize while stirring, and then inject nitrogen gas at a pressure of 0.02Mpa.
[0069] Add oxygen-expelling water for injection at a temperature of 90°C to 100°C in the concentrated preparation tank, and the amount of added oxygen-expelling water for injection is 80% of the prescribed amount; Dissolved sodium chloride; predissolve the prescribed amount of cysteine hydrochloride in a glass container with 60°C oxygen-expelling water for injection, and the concentration of the cysteine hydrochloride solution is 30 mg / ml; then add the prescribed amount of Edala Bong was added to the cysteine hydrochloride solution for wetting to obtain solution I.
[0070] 1.2 Add the so...
Embodiment 2
[0081] Edaravone injection was prepared according to the original research prescription of Edaravone injection in Table 1 above. The preparation method is:
[0082] 1. Concentrated
[0083] 1.1 Preparation of oxygen-expelling water for injection: put water for injection at 90°C to 100°C in a container, vacuumize while stirring, and then inject nitrogen gas at a pressure of 0.02Mpa.
[0084]Add oxygen-expelling water for injection at a temperature of 90°C to 100°C in the concentrated preparation tank, and the amount of added oxygen-expelling water for injection is 70% of the prescription amount; cool down the temperature of oxygen-expelling water for injection to 80°C, Dissolved sodium chloride; pre-dissolve the prescribed amount of cysteine hydrochloride in a glass container with 80°C deoxygenation water for injection, and the concentration of the cysteine hydrochloride solution is 32mg / ml; then add the prescribed amount of Edala Bong was added to the cysteine hydrochl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com