Cinnamic acetal diglycidyl ether and preparation method and application thereof
A technology of diglycidyl ether and glycidyl ether, which is applied in the field of cinnamon acetal diglycidyl ether and its preparation, can solve the problems of restricting the application of polymer materials, and achieve a low difficulty, wide application prospect, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
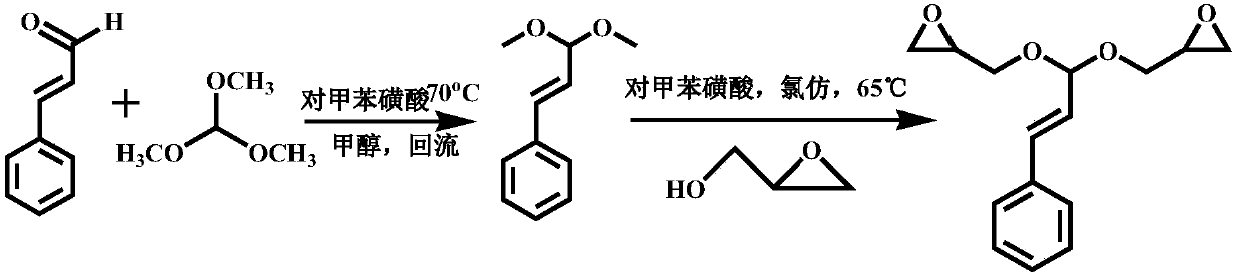
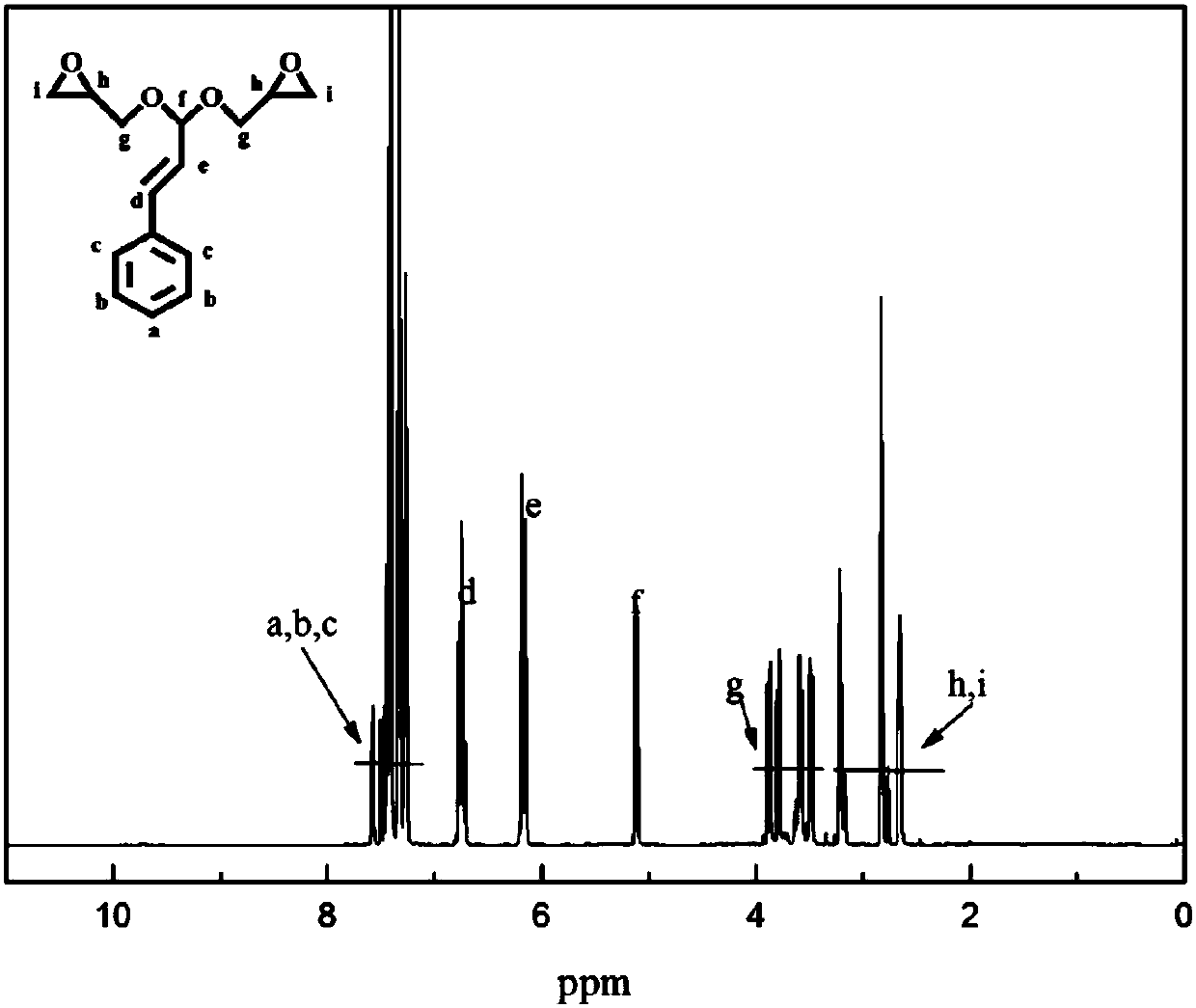
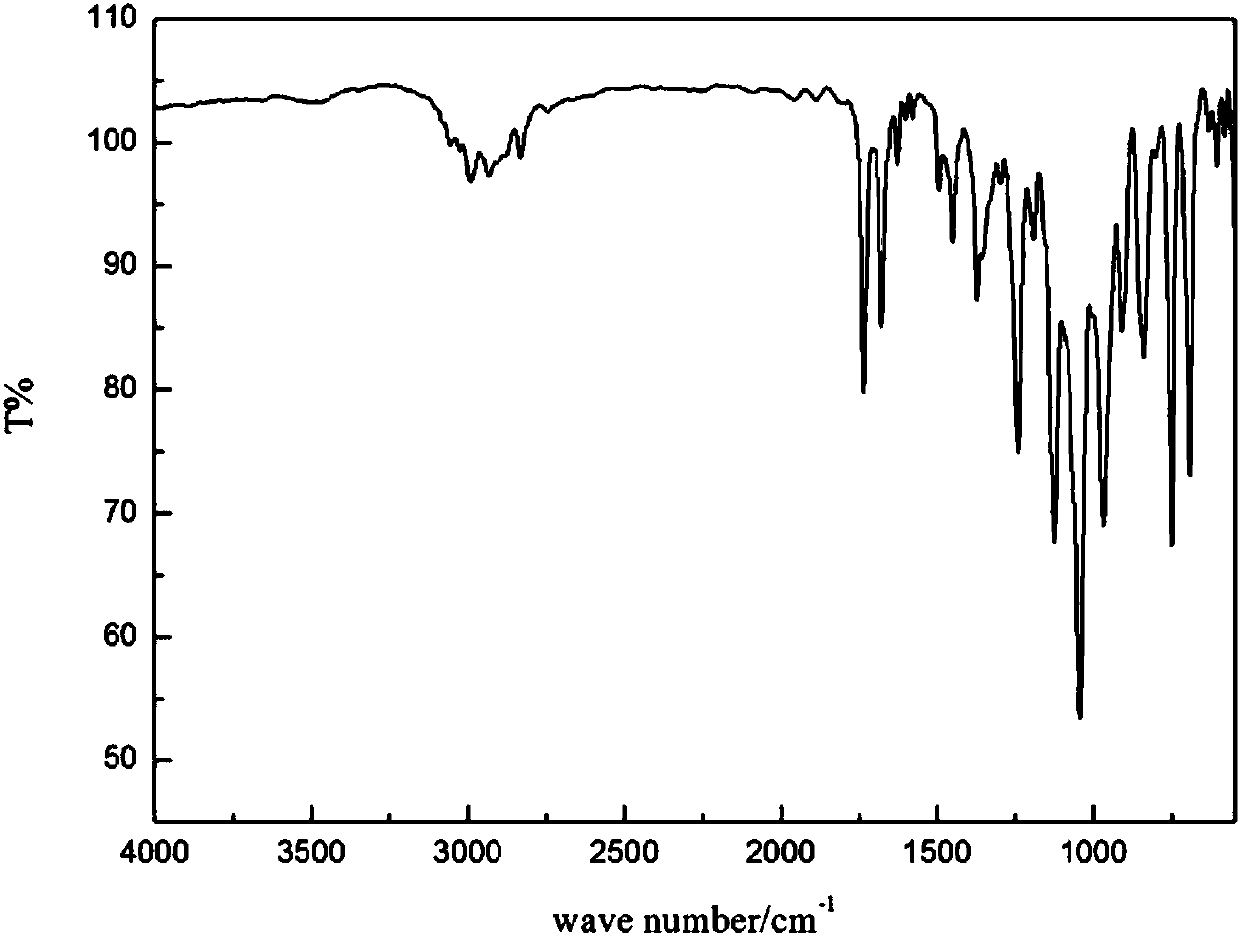
[0032] Such as Figure 1 to Figure 3 Shown, in embodiment 1 of the present invention, comprise the following steps:
[0033] (1) At room temperature, weigh 6.6g (0.05mol) of cinnamaldehyde and 23.8g (0.22mol) of trimethyl orthoformate in a dry and clean round bottom flask, dissolve them in 60mL of methanol, add 30mg of p-toluenesulfonic acid , the reaction system was refluxed at 72°C and 800r / min for 6 hours;
[0034] (2) Add saturated aqueous sodium bicarbonate solution to the reaction system in batches to pH = 7‐8, remove p-toluenesulfonic acid, concentrate under reduced pressure, extract 3 times with 3*40mL dichloromethane, collect the organic layer, add 8g of anhydrous Sodium sulfate dehydration for 6 hours;
[0035] (3) Remove anhydrous sodium sulfate by filtration, remove the solvent under reduced pressure, and separate by column chromatography. The volume ratio of the column chromatography components is petroleum ether: ethyl acetate=10:1, and the light yellow liquid ...
Embodiment 2
[0045](1) At room temperature, weigh 3.3g of cinnamaldehyde and 9.9g of trimethyl orthoformate in a dry and clean round bottom flask, dissolve them in 30mL of methanol, add 12mg of p-toluenesulfonic acid, and the reaction system is at 68°C, 800r / min under reflux for 4 hours;
[0046] (2) Add saturated aqueous sodium bicarbonate solution to the reaction system in batches to pH = 7-8, remove p-toluenesulfonic acid, concentrate under reduced pressure, extract 3 times with 3*20mL dichloromethane, collect the organic layer, add 4g of anhydrous Sodium sulfate dehydration for 4 hours;
[0047] (3) Remove anhydrous sodium sulfate by filtration, remove the solvent under reduced pressure, and separate by column chromatography. The volume ratio of the column chromatography components is petroleum ether: ethyl acetate=10:1, and the light yellow liquid cinnamon acetal 3.87 is obtained. g, yield 87%;
[0048] (4) Dissolve 3.87g of cinnamon acetal and 3.48g of glycidyl ether obtained in s...
Embodiment 3
[0052] (1) At room temperature, weigh 5g of cinnamaldehyde and 25g of trimethyl orthoformate in a dry and clean round bottom flask, dissolve them in 90mL of methanol, add 45mg of p-toluenesulfonic acid, and the reaction system is at 80°C, 800r / min Under reflux reaction for 8 hours;
[0053] (2) Add saturated aqueous sodium bicarbonate solution to the reaction system in batches to pH = 7‐8, remove p-toluenesulfonic acid, concentrate under reduced pressure, extract 3 times with 3*30mL dichloromethane, collect the organic layer, add 10g of anhydrous Sodium sulfate dehydration for 6 hours;
[0054] (3) Remove anhydrous sodium sulfate by filtration, remove the solvent under reduced pressure, and separate by column chromatography. The volume ratio of the column chromatography components is petroleum ether: ethyl acetate=10:1, and the light yellow liquid cinnamon acetal 5.2 is obtained. g, yield 77%;
[0055] (4) Dissolve 5.2g of cinnamon acetal and 6.24g of hydroxyethyl acrylate o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com