Ganmaoling granules and preparation method thereof
A technology of Ganmaoling and granules, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems affecting quality and curative effect, deliquescence, softening and deterioration of Ganmaoqingre granules, etc. Achieve the effect of pneumonia inhibition and death protection, low hygroscopicity, and internal quality improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 A Ganmaoling Granule
[0032] Described Ganmaoling granules consist of the following components and parts by weight thereof:
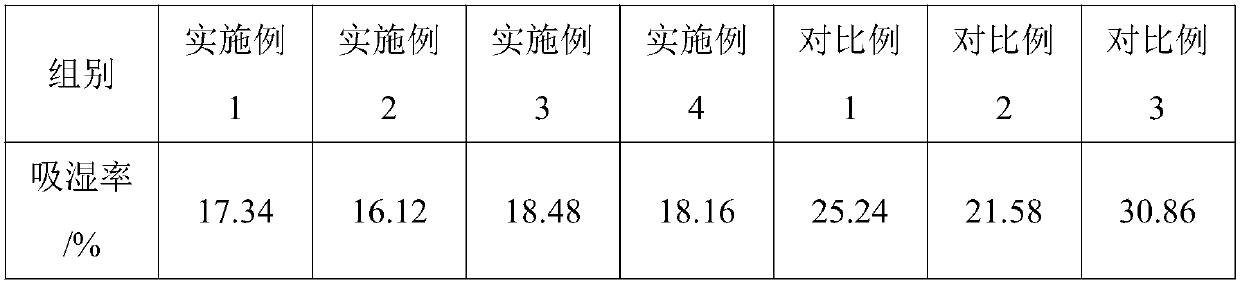
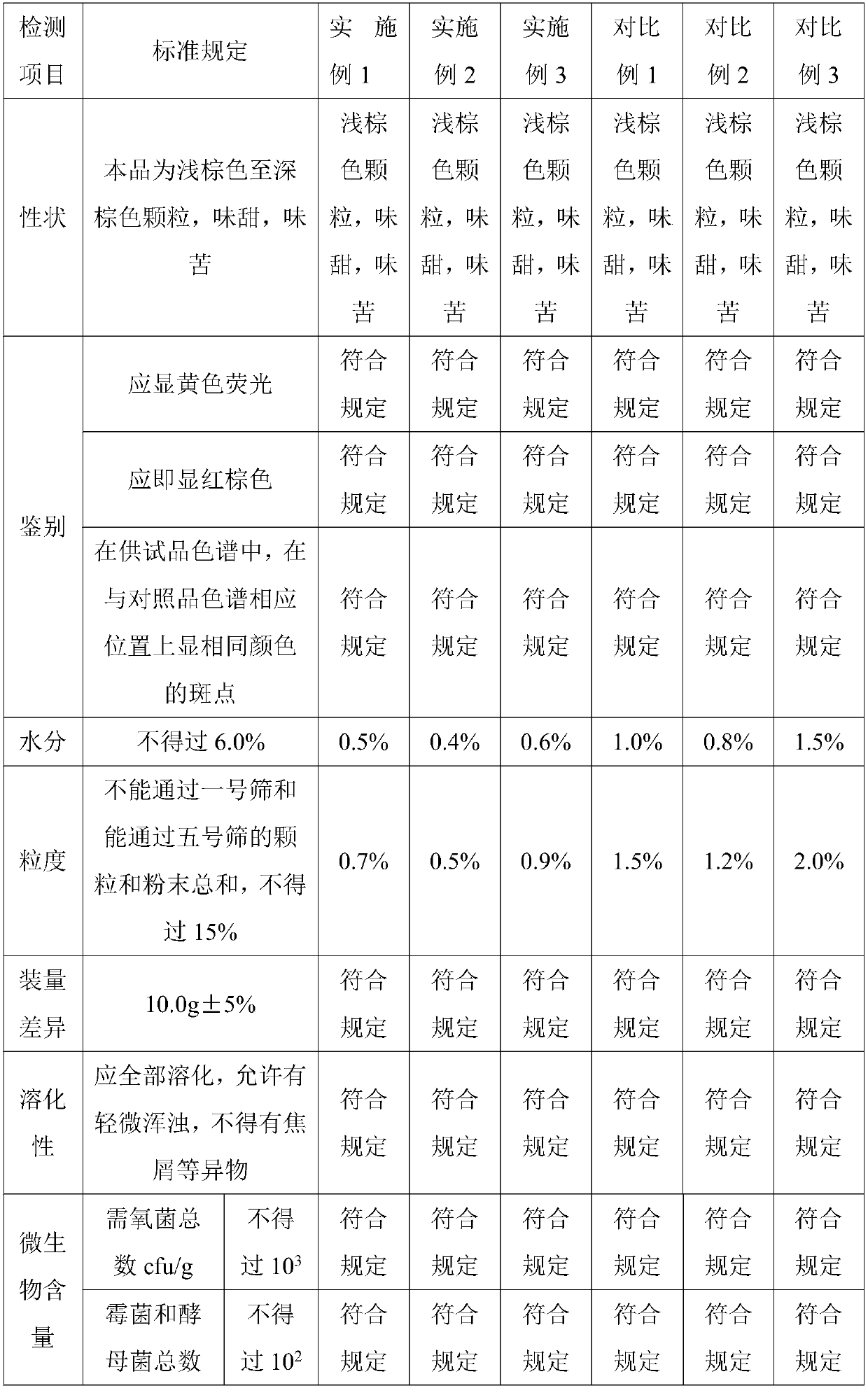
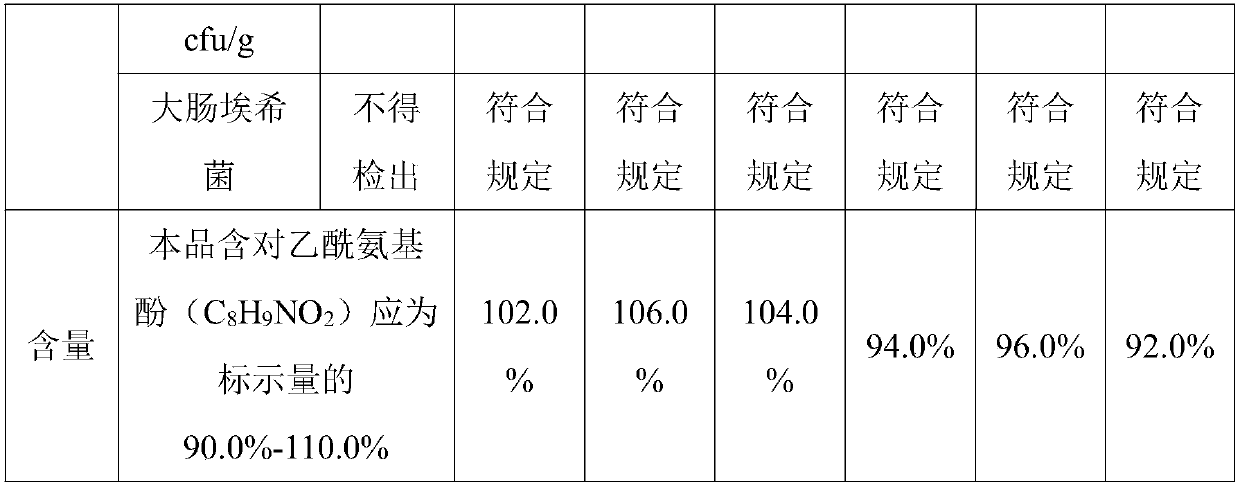
[0033] 450 parts of Sanchaku, 300 parts of calendula silver plate, 200 parts of wild chrysanthemum, 700 parts of gang plum, 15 parts of acetaminophen, 0.1 part of chlorpheniramine maleate, 0.2 part of caffeine, 0.1 part of peppermint oil, lactose 200 parts, 100 parts of demyelinated starch, 60 parts of microcrystalline cellulose, 5 parts of β-cyclodextrin, 3 parts of polyvinylpyrrolidone and 80 parts of ethanol.
[0034] The preparation method of described debranched starch is:
[0035] (1) Take 1g of cornstarch, add it to 10mL of water, stir evenly, heat in a water bath to 60°C, stir and add 50mL of dimethyl sulfoxide, heat to boiling, and boil for 1 hour to obtain a solution;
[0036] (2) Cool the solution obtained in step (1) to room temperature, add 6 times its volume of ethanol, and centrifuge to obtain a precipitate;
[0037] ...
Embodiment 2
[0044] Example 2 A Ganmaoling Granule
[0045] Described Ganmaoling granules consist of the following components and parts by weight thereof:
[0046] 490 parts of Sanchaku, 325 parts of calendula silver plate, 250 parts of wild chrysanthemum, 740 parts of gang plum, 20 parts of acetaminophen, 0.4 part of chlorpheniramine maleate, 0.4 part of caffeine, 0.2 part of peppermint oil, lactose 250 parts, 150 parts of amylopectin, 90 parts of microcrystalline cellulose, 7.5 parts of β-cyclodextrin, 4 parts of polyvinylpyrrolidone and 100 parts of ethanol.
[0047] The preparation method of the debranched starch is similar to Example 1.
[0048] Preparation:
[0049] S1 Mix Sanchaku, Marigold Silver Plate, Wild Chrysanthemum, and Gangmei evenly to obtain medicinal materials;
[0050] S2 Add 7 times the weight of water to the medicinal material obtained in step S1, decoct twice for 2.5 hours each time, combine the decoction, filter, concentrate under reduced pressure, and spray dry to...
Embodiment 3
[0054] Example 3 A Ganmaoling Granule
[0055] Described Ganmaoling granules consist of the following components and parts by weight thereof:
[0056] 530 parts of Sanchaku, 350 parts of calendula silver plate, 300 parts of wild chrysanthemum, 780 parts of gang plum, 25 parts of acetaminophen, 0.7 part of chlorpheniramine maleate, 0.6 part of caffeine, 0.3 part of peppermint oil, lactose 300 parts, 200 parts of demyelinated starch, 120 parts of microcrystalline cellulose, 10 parts of β-cyclodextrin, 5 parts of polyvinylpyrrolidone and 120 parts of ethanol.
[0057] The preparation method of the debranched starch is similar to Example 1.
[0058] Preparation:
[0059] S1 Mix Sanchaku, Marigold Silver Plate, Wild Chrysanthemum, and Gangmei evenly to obtain medicinal materials;
[0060] S2 Add 8 times the weight of water to the medicinal material obtained in step S1, decoct twice for 3 hours each time, combine the decoction, filter, concentrate under reduced pressure, and spra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com