Cholesterol pyrophosphate, and preparation method and use thereof
A pyrophosphorylated cholesterol and chemical structural formula technology, which is applied in the field of medicine, can solve problems affecting human health, achieve the effect of improving treatment effect and improving research progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. At 0°C, dissolve cholesterol (1.933g, 5mmol) in 40mL of anhydrous dichloromethane, then slowly add triethylamine (1.125mL, 7.5mmol), DMAP (60mg, 0.5mmol), and finally slowly add Tosyl chloride (1.43g, 7.5mmol), stirred overnight at room temperature. Wash twice with 1mol / L hydrochloric acid, 40mL each time, and wash twice with saturated NaCl solution, 40mL each time. An appropriate amount of anhydrous Na was added to the organic layer 2 SO 4 dry. The organic solvent was evaporated by vacuum rotation. Pass through a silica gel column (petroleum ether: ethyl acetate 4:1).
[0040] 2. Product 2, (2.16g, 4mmol) was dissolved in 30mL of dry dioxane, and (15mL, 111mmol) triethylene glycol was added. (TLC identification of reaction endpoints). The organic solvent was evaporated, the reactant was dissolved in 50mL chloroform, saturated NaHCO 3 The solution was extracted twice, 50mL each time, and washed twice with saturated NaCl solution, 50mL each time. Appropriate a...
Embodiment 2
[0050] (1), 2,2-bis-(bromomethyl)propane-1,3-diol and an appropriate amount of sodium azide were dissolved in dimethylformamide, stirred overnight at 120°C, filtered, and the organic solvent was removed, the residue The standard ether / NaCl aqueous solution was extracted, the crude product was passed through a silica gel column, and eluted with chloroform:methanol=20:1, and further purified with bromoacetic acid (1:1.3, mol / mol) by adding an appropriate amount of anhydrous di in methyl chloride. Add 1.1 equivalents of dicyclohexylcarbodiimide (DCC) and 0.1 equivalents of 4-dimethylaminopyridine (DMAP) to the above solution at zero temperature, stir at room temperature for 4 hours, filter, evaporate to dryness under reduced pressure, and pass through a silica gel column (cyclohexane: Ethyl acetate 3:1) to obtain product 1.
[0051] (2), 1 equivalent of potassium tri(tetrabutylammonium) hydrogen pyrophosphate and 10.5 equivalents of the crude product were dissolved in an appropr...
Embodiment 3
[0059] The synthetic route is as follows:
[0060]
[0061] Synthesis of Bis-L-Phosphoserine Polypropylene Glycol Ethylene Oxide Polymer (a) and Pyrophosphate Polypropylene Glycol Ethylene Oxide Polymer (b)
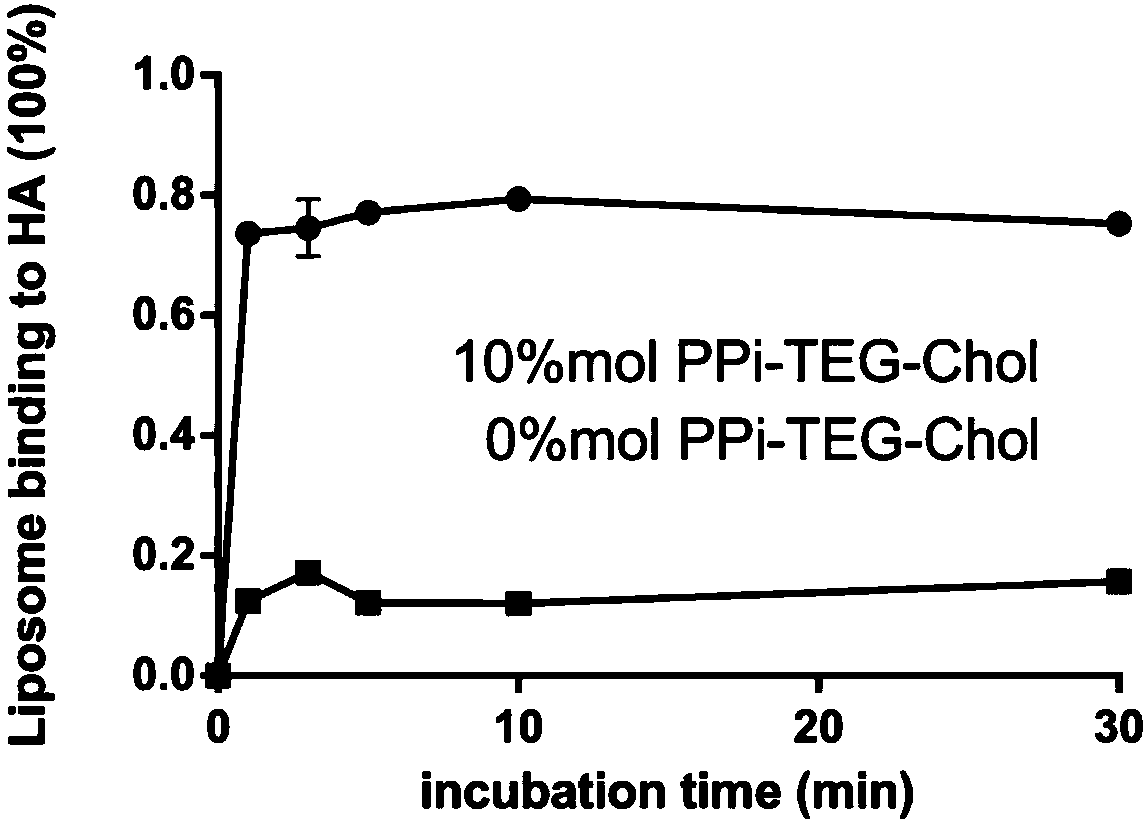
[0062] Targeting HA kinetics in vitro:
[0063] Targeting kinetics of tooth-targeting polymeric micelles on HA with Figure 4 It can be seen that the targeting ability of tooth-targeting polymeric micelles (DPS-TBM) prepared with bis-L-phosphoserine polypropylene glycol ethylene oxide addition polymer is not as good as that of pyrophosphate polypropylene glycol ethylene oxide addition polymer. Teeth-targeting polymeric micelles (PPi-TBM) prepared from
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com