Intraocular drug delivery composition and preparation method thereof
A composition and drug delivery technology, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve the problems of low eye bioavailability, short residence time, and failure to reach the posterior segment of the eye, etc., to achieve Improve the efficiency of membrane penetration and eye bioavailability, the preparation method is simple, and the effect of promoting rapid penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
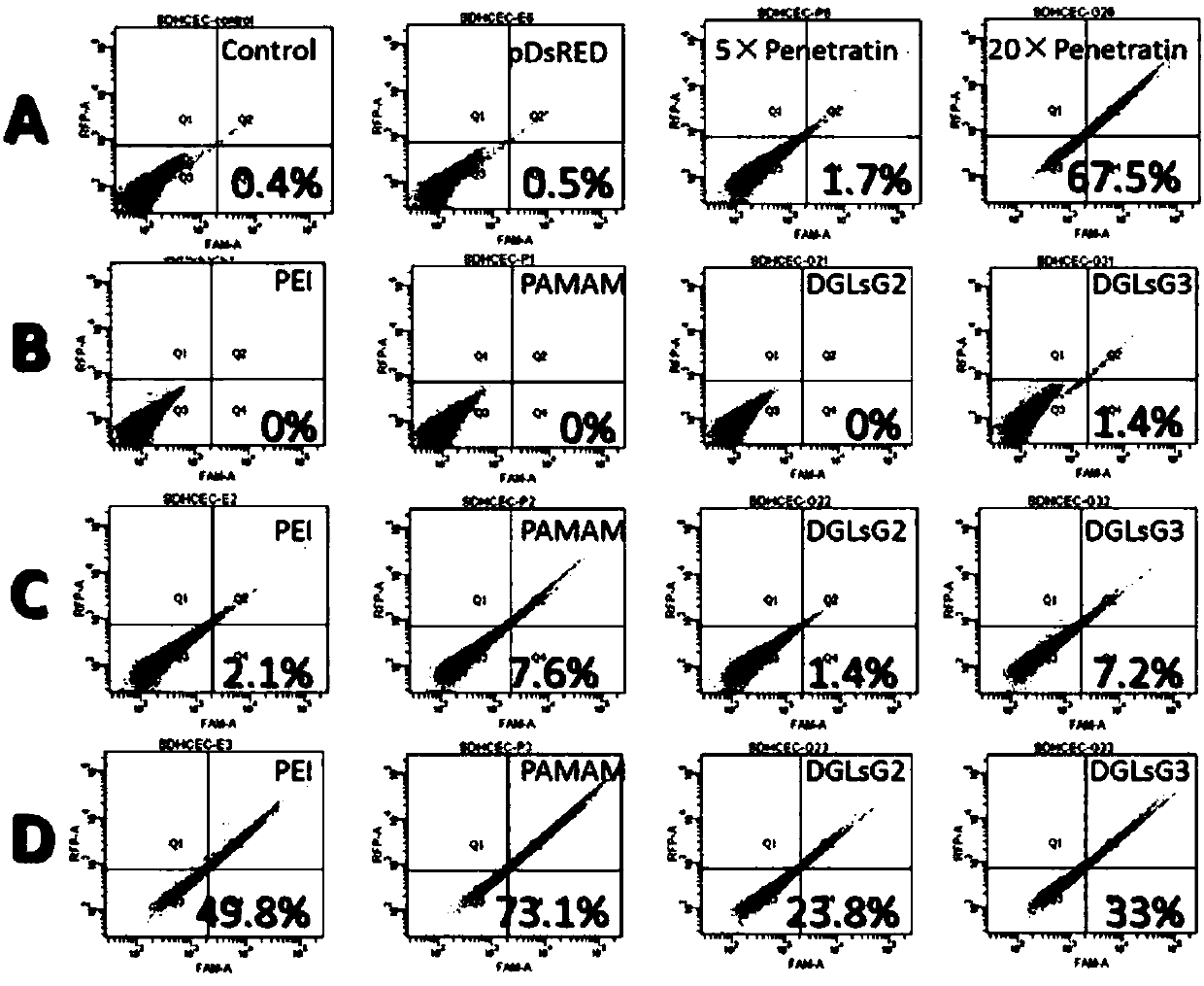
[0035] The preparation of the primary composition pRFP / BCP using branched cationic polymers (BCP) to encapsulate red fluorescent protein particles (pRFP): different concentrations of polyethyleneimine (PEI), polylysine (DGLs) Mix with polyamide-amine dendrimer (PAMAM) solution in equal volume with 0.05 mg / ml plasmid DNA solution according to a certain charge ratio (nitrogen / phosphorus ratio), vortex for 30 s, and place at room temperature for 30 min to obtain freshly prepared different Primary composition solution for charge ratio (nitrogen / phosphorus ratio);
[0036] Preparation of surface-modified penetrating peptide (CPP) composition pRFP / BCP / CPP: The pRFP / BCP primary composition prepared above was mixed with different concentrations of TAT, Penetratin, oligo-arginine short peptide Poly(arginine) n (where n=6-12), low molecular weight protamine (LMW Protamine), Transportan, VP-22, HCT (9-32) and Pvec solution are mixed in equal volumes according to a certain charge ratio, v...
Embodiment 2
[0038] Use agarose gel electrophoresis (AGE) to screen the ratio of the three components in the composition described in Example 1, which are respectively red fluorescent protein particles (pRFP), branched cationic polymers (PEI, DGLs and PAMAM ) and penetrating peptides (TAT, Penetratin, Poly(arginine) n where n=6-12, LMWProtamine, Transportan, VP-22, HCT(9-32) and Pvec). Observe DNA bands under UV light and take pictures;
[0039] The results showed that the ability of branched cationic polymer PEI to form complexes with pRFP and CPP was poor, while the complexes formed by DGLs and PAMAM were stable when the charge ratio of pRFP was 1:3-10:1. PAMAM with a molecular weight between 2,000-200,000 can form a primary complex with pRFP, and the preferred molecular weight range is 5,000-50,000. DGLs with a molecular weight between 5,000 and 200,000 can form primary complexes with pRFP, and the preferred molecular weight range is 8,000 to 80,000. A stable secondary complex can be...
Embodiment 3
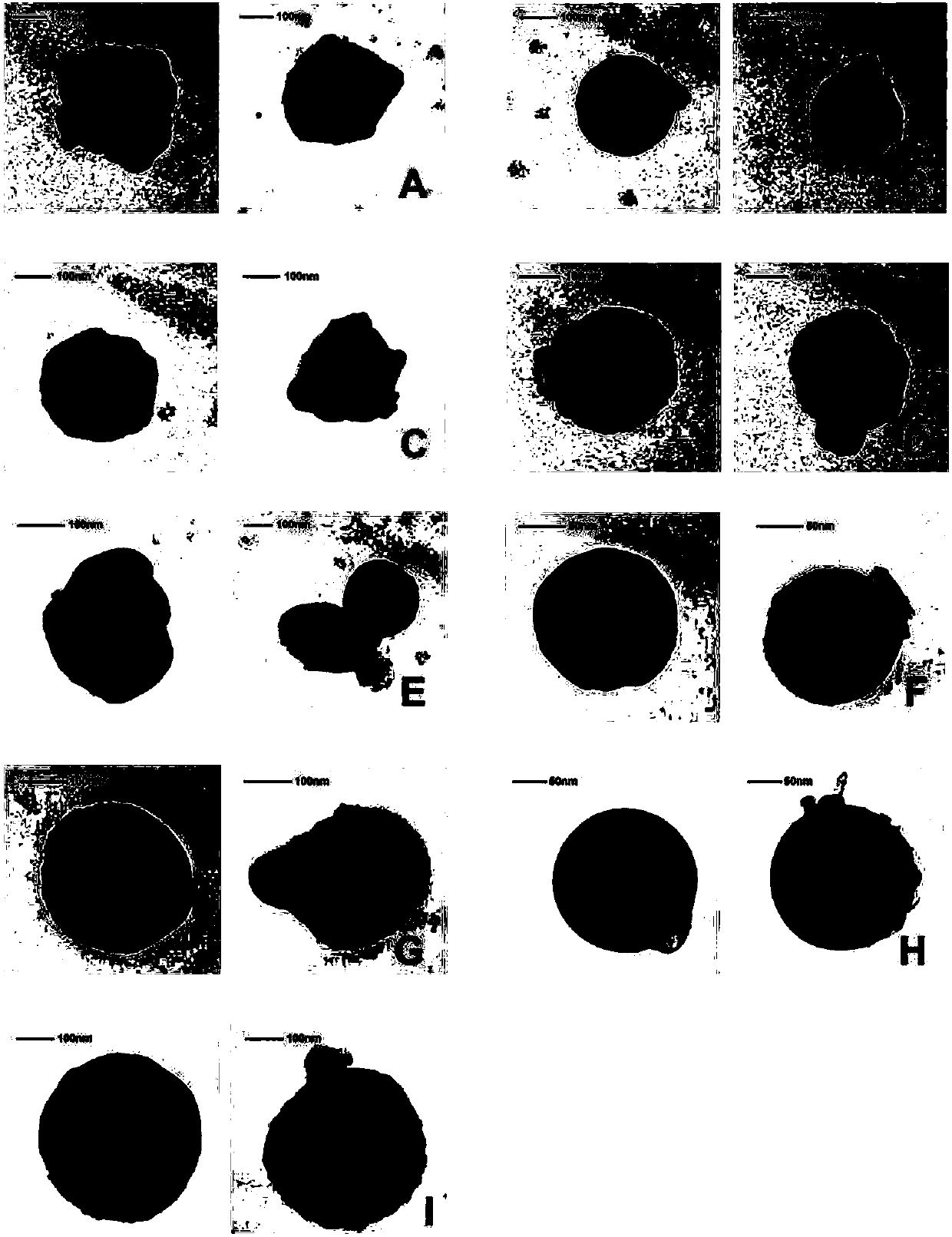
[0041] According to the formulation screening results, the composition solution was prepared; the particle size and polydispersity index (PDI) of each sample were quantitatively measured with a dynamic light scattering particle size analyzer (Malvern Zetasizer Nano S). Taking the complex pRFP / BCP / Penetratin formed by red fluorescent protein particles, branched cationic polymers and Penetratin as an example, the measurement results are shown in Table 1 below:
[0042] Table 1. Particle size distribution table of pRFP / BCP / Penetratin composition
[0043]
[0044] Note: P20 means that the charge ratio of Penetratin to pRFP in the prescription is 20:1; DGLsG2 means the 2nd generation DGLs with a molecular weight of 8600; DGLsG3 means the 3rd generation DGLs with a molecular weight of 22000;
[0045] Adopt transmission electron microscope to carry out morphological detection to composition sample, the result is as attached figure 1 As shown, the shape of the complex is a solid s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com