A crystalline form of malate salt of a tyrosine kinase inhibitor and a preparation method thereof
A technology of crystallization and interplanar spacing, applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as instability of crystal form I, achieve good crystal form stability, and the production process is repeatable and reproducible. control, the effect of stable production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
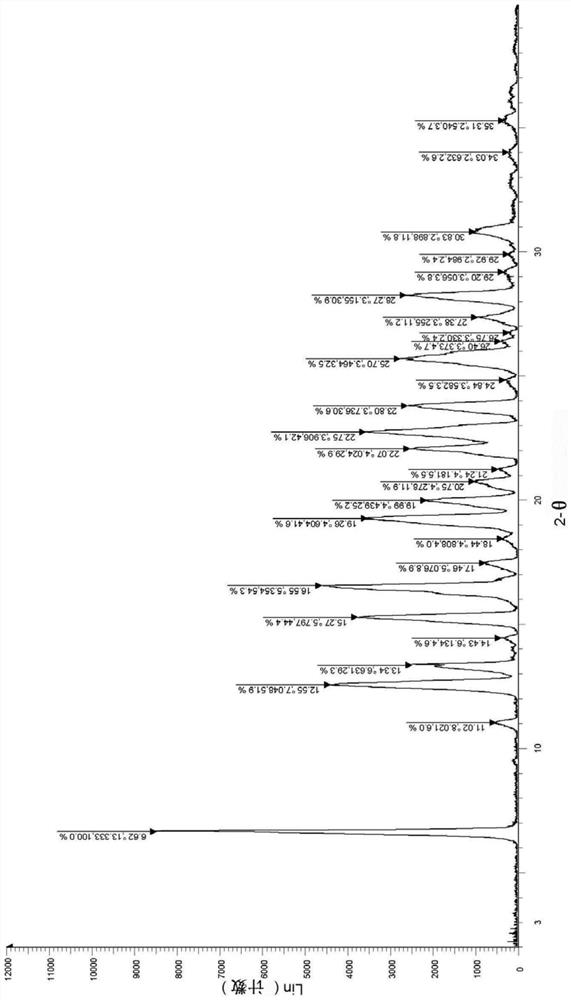
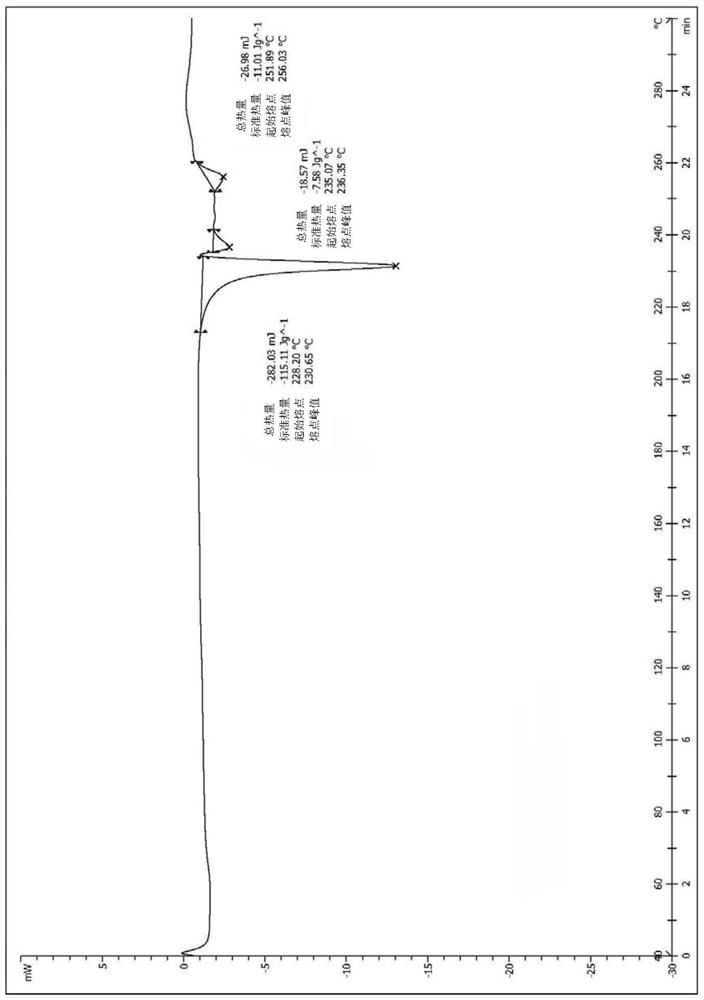
[0037] Take (1.0g, 1.84mmol) the compound represented by formula (I) (prepared according to the method disclosed in WO2007085188) and add it to a 250ml single-necked bottle, add 208ml of ethanol, heat to dissolve, then cool to room temperature, stand for crystallization, and suction filter , dried in vacuo to obtain 0.57 g of solid, with a yield of 57.0%. The X-ray diffraction spectrum figure of this crystalline sample is shown in figure 1 . The crystallization at about 6.62 (13.33), 11.02 (8.02), 12.55 (7.05), 13.34 (6.63), 15.27 (5.80), 16.55 (5.35), 17.46 (5.08), 19.26 (4.60), 19.99 (4.44), 20.75 There are characteristic peaks at (4.28), 22.07(4.02), 22.75(3.91), 23.80(3.74), 25.70(3.46), 27.38(3.26), 28.27(3.16) and 30.83(2.90). See the DSC spectrum figure 2 , with a melting endothermic peak at 230.65°C, this crystal form is defined as II crystal form.
Embodiment 2
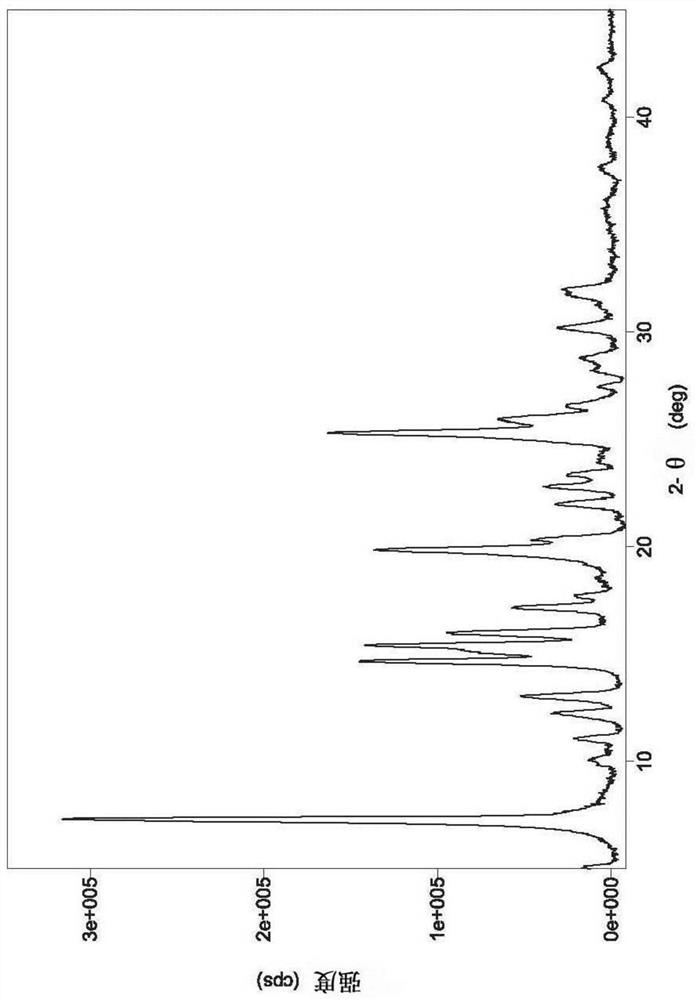
[0039] Take (1.0g, 1.84mmol) the compound represented by formula (I) (prepared according to the method disclosed in WO2007085188) and add it to a 250ml single-necked bottle, add 50ml ethanol and 50ml water, heat to dissolve, then cool to room temperature, and stand for crystallization , suction filtration, and vacuum drying to obtain 0.64 g of solid, with a yield of 64.0%. The X-ray diffraction spectrum figure of this crystalline sample is shown in image 3, the peak list of the X-ray diffraction spectrum is shown in Table 1. The crystallization at about 7.34 (12.02), 10.09 (8.76), 11.06 (7.99), 12.25 (7.22), 13.03 (6.79), 14.69 (6.02), 15.11 (5.86), 15.42 (5.74), 16.00 (5.54), 17.17 (5.16), 17.72(5.00), 19.86(4.47), 20.35(4.36), 21.96(4.04), 22.77(3.90), 23.37(3.80), 25.33(3.51), 25.96(3.43), 26.52(3.36), 28.77 There are characteristic peaks at (3.10), 30.17 (2.96) and 31.65 (2.83). See the DSC spectrum Figure 4 , with a sharp melting endothermic peak at 240.64°C, this c...
Embodiment 3
[0043] Take (1.0g, 1.84mmol) the compound represented by formula (I) (prepared according to the method disclosed in WO2007085188) and add it to a 250ml single-necked bottle, add 90ml methanol, heat to dissolve, then cool to room temperature, stand for crystallization, and suction filter , dried in vacuo to obtain 0.45 g of an orange-yellow solid compound represented by formula (I), with a yield of 45.0%. Its X-ray powder diffraction and DSC patterns are researched and compared, and it is determined that the product is in the II crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com